Abstract
With the emergence of new applications for low-melting-point solder alloys, such as self-healing and semisolid additive manufacturing, understanding their long-term microstructural stability has become necessary. Sn-Bi-In alloys provide an attractive option for such applications, with a melting point below 150°C; however, little is known about their long-term stability and microstructural evolution. In this research, we conducted extended stability experiments for Sn-40Bi and Sn-40Bi-10In alloy compositions over three different heat-treatment periods of 12 h, 168 h, and 360 h. The microstructure of the two alloys prominently featured Sn-rich globules, which were observed to be finer in the Sn-40Bi-10In alloy than in the Sn-40Bi composition. The finer globules in Sn-40Bi-10In may be attributed to initial Sn-rich islands, which facilitated improved globule refinement during the semisolid stage. Furthermore, both the size and fraction of the globules were found to increase with increasing heat treatment time in Sn-Bi-In alloy; it was also observed that the Sn-40Bi-10In alloy globules exhibited a gradual rise in overall Bi content with time, whereas the interglobular Sn/Bi/BiIn eutectic regions showed a decrease in Bi content and an increase in Sn content.
Similar content being viewed by others
Introduction
With the microelectronics industry’s transition to lead-free solder materials and the trend toward more compact and integrated devices, the demand for reliable solder connections has increased substantially. Solder joint failures commonly result from fatigue and are typically instigated by localized strains induced by thermomechanical stresses. These effects give rise to the initiation of cracks, their subsequent propagation, and solder joint failure. Under such conditions, using a low-temperature soldering process with a reflow temperature below 160°C may be beneficial.1 As a result, Sn-Bi-In alloys are suitable for low-temperature applications2,3,4 and have garnered industry interest. Furthermore, incorporating In into Sn-Bi alloys can enhance heat dissipation in electronic systems with high power density.5
Using temperature variation to heal solder material, particularly in Sn-Bi alloys, is a novel approach recently explored to enhance solder joint reliability under thermomechanical stresses.6,7,8 Sn-Bi alloys have been shown to exhibit a liquid-assisted healing phenomenon when subjected to compressive forces and elevated temperatures. This self-healing process involves localized melting6,7; an alloy must possess a two-phase region where both the solid and liquid phases can coexist to enable this healing mechanism. The effectiveness of damage repair hinges on the presence and morphology of the liquid phase.8 Harnessing the potential of self-healing solder joints could extend their service life, enhancing reliability, minimizing sudden failures, and reducing electronic waste. Given the excellent outcomes with Sn-Bi, it is crucial to explore Sn-Bi-In alloys, since the addition of In has been shown to increase ductility and reduce the alloy’s melting point.9,10,11
As the solder joint and the bulk alloy heat up at high temperatures, it becomes important to quantify microstructural stability to ensure that the self-healing process does not deteriorate the properties of the solder joint. The problem of microstructural stability in solder alloys has been widely studied by various authors.12,13,14,15 Anderson et al.12 demonstrated how the addition of Co to Sn-Ag-Cu alloys not only led to an apparent solidification catalyst effect, resulting in improved microstructural refinement, but also played a role in enhancing microstructural stability and retaining strength by altering the coarsening behavior of solder joints during high-temperature aging. Extensive research has been conducted on the influence of element doping on the microstructural stability of Sn-Cu alloys at high temperatures.13,14,15
Despite some research on the precipitation of Bi in Sn-rich solder alloys aged at room temperature in the literature,16,17,18,19,20 there remain limited data on the coarsening of Bi-phase at higher temperatures. Few studies have investigated the coarsening of Bi particles in SAC-Bi/Cu, Sn-Ag-Bi/Cu, and Sn-Bi/Cu joints aged at room temperature. Belyakov et al.17 reported the growth of Bi plates over aging time. Wu et al.18 extensively investigated the microstructural evolution, particularly the coarsening process, in SAC-3Bi solder alloys during room-temperature aging. They demonstrated that the precipitation of Bi is a surface diffusion phenomenon, substantiating this by eliminating the surface, confirming the absence of Bi particles, and observing a “reset” in the precipitation process. The study also involved computing precipitation rates for various microstructures, revealing that the initial microstructural state, influenced by the solder cooling rate during reflow, significantly shapes the progression of precipitation and the corresponding precipitation rate.
In this study, a comprehensive investigation was carried out to understand the microstructural changes, particularly coarsening, in a Sn-Bi-In solder alloy during semisolid heat treatment. Samples of Sn-Bi-In solder were created and subsequently heat treated to study the coarsening and phase distribution mechanisms in such alloy compositions. The findings suggest that Bi precipitation is influenced by the increased Bi content within the globular Sn-rich matrix, the presence of In in solution, and the size of the globules, which, in turn, is affected by the cooling rate. Furthermore, statistics on particle count and particle area fractions for the microstructures were quantified, highlighting the substantial impacts of the In addition and initial microstructure on the progression of Bi precipitation within the globules. Coarsening and growth mechanisms of the Sn/Bi/BiIn ternary eutectic regions were also examined.
Materials and Methods
The Sn-40Bi and Sn-40Bi-10In (in wt.%) alloys were prepared by casting them in a graphite mold. The elements used in the alloys were high-purity Sn (99.98 wt.%), Bi (99.99 wt.%), and In-5 wt.% Sn pre-alloy. The material was melted in an induction furnace (Power-Trak 50-30R, Inductotherm VIP, USA) and poured into a high-density graphite mold. After solidification, cylinders with a diameter of 4 mm and height of 4 mm were machined from the cast material and placed on the top of a Cu plate (polished sequentially with SiC paper up to a 1200 mesh surface finish) to be soldered in a goniometer (Kruss DSHAT HTM Reetz GmbH, Berlin, Germany). Using this system, samples of Cu/Sn-40Bi and Cu/Sn-40Bi-10In solder joints were prepared.
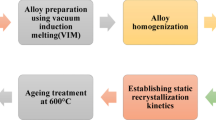
Figure 1 shows a schematic of the process followed for preparing the alloys, the solder joints, and aging. The solder joints were produced using a goniometer under an argon atmosphere. The solders were heated at 10°C/min up to 180°C and maintained for 0.25 h. After the 0.25-h period, the samples were slowly cooled inside the furnace. Subsequently, the solder joints were machined into square cross-section pillars with an edge length of 1.0 mm and subjected to semisolid heat treatments (aging). Six samples were prepared and subjected to different heat treatments to study the regions formed during semisolid heat treatment and assess the impact of Bi precipitation. Table I shows a summary of the samples used in this study. All treated samples were water-quenched after aging, followed by scanning electron microscopy (SEM) and energy-dispersive x-ray spectroscopy (EDS) analyses to quantify the evolution of phase morphology and compositional heterogeneity of the sample with aging.
Diagram illustrating the process of alloy preparation, followed by a reflow operation conducted within a goniometer to produce solder joints in a copper plate. Subsequently, the process involves machining square section pillars measuring 1.0 mm × 1.0 mm and heat treatment above the solidus temperature.
Note that the two alloy compositions were treated under equivalent conditions with respect to the solidification range, as demonstrated in Fig. 2. Both alloys were treated at a temperature T, above the solidus temperature Ts, such that the difference between T and Ts was 33% of the solidification range ΔT (i.e., T − Ts = 0.33 × ΔT).
After aging and quenching of the samples, the solder joints were mounted in epoxy and polished to achieve a smooth surface. The samples were first polished using 600-grit paper (coarse and fine), then 1200-grit paper (coarse and fine), and finally with alumina and colloidal silica to achieve a fine finish of 0.05 μm. Immediately after preparation, the samples were imaged with an FEI Quanta 3D FEG (FEI Company, Hillsboro, OR, USA) SEM using the backscattered electron (BSE) detector. An accelerating voltage of 20 kV, spot 1.0, and a working distance of ~ 10 mm were used during imaging. In tandem, EDS was also carried out on the samples with an Oxford INCA Xstream-2 with an X-Max 80 detector (Oxford Instruments, Peabody, MA, USA). The microstructure and composition of the alloys were characterized at three locations in the globular and three locations in the interglobular regions across the cross-section. SEM and EDS data from each location were analyzed using ImageJ (Bethesda, MD, USA) and Avizo software (Thermo Fisher Scientific, Waltham, MA, USA) to obtain phase fraction data in the eutectic zones. Simple grayscale-based thresholding was applied to the Z-contrast images obtained from SEM imaging to separate the globules and Bi particles of interest from the surrounding microstructure.
Results and Discussion
Evolution of Microstructure with Heat Treatment
Figure 3 shows the evolution of the microstructure as a function of semisolid treatment. SEM images and EDS reveal the existence of two distinct phases, β-Sn and Bi, in the Sn-40Bi alloy (see Fig. 3e and f) and three phases, β-Sn, Bi, and BiIn, in the Sn-40Bi-10In alloy (Fig. 3a, b, c, and d). This agrees with the CALPHAD (CALculation of PHAse Diagrams) predictions shown in Fig. 2.
In the as-reflowed condition (Fig. 3a and e), the initial microstructures of the Sn-40Bi-10In alloy show the presence of Sn-rich islands, whereas in the Sn-40Bi, the growth pattern consists of Sn-rich dendrites. After semisolid aging, two clear regions were observed in the Sn-Bi-In alloy. As shown in Fig. 3b, c, and d, Sn-rich “globules” appear with In and Bi in solution and embedded Bi particles. Surrounding the Sn-rich globules were interglobular ternary eutectic zones. The untreated Sn-Bi-In samples (Fig. 3a) exhibited a notably coarse Sn-Bi eutectic structure, whereas the interglobular zones of the treated samples (Fig. 3b, c, and d) primarily showed the prevalence of a finer ternary eutectic microstructure.
Based on the global EDS dot-maps, it can be observed that the BiIn phase is predominantly situated at the boundaries between the Sn-rich regions and the eutectic, which is strikingly different from the untreated, as-reflowed Sn-Bi-In sample. Furthermore, the BiIn phase exhibits minor contact with the coarse Bi lamellar particles within the eutectic region. The relative separation of the BiIn phase during eutectic formation could offer advantages in facilitating the initial melting process during semisolid treatment since this phase melts congruently at 110°C.21 Bi precipitation was also observed in Sn-rich island-like structures at the 0 h mark and in globules at 12 h, 168 h, and 360 h. Additionally, the presence of β-Sn dendrites within the interglobular region was particularly noticeable in the Sn-Bi-In samples after 168 h and 360 h.
As is evident from Fig. 3, the transformation from dendritic structures into more spherical or ellipsoidal and denser globules occurs during the isothermal resting period of the solid + liquid mixture. Over time, these rounded particles exhibit enhanced mobility as they move alongside one another, as described by Flemmings.22 The diameter of these globules, at a specific point during semisolid treatment, tends to be roughly equivalent to the size the dendrites would attain in an untreated melt, as depicted in Fig. 3e and f. In Sn-Bi-In samples, the absence of dendritic structures at the interface of the globules appears to advance the formation of these globules, especially evident in the rounded original Sn-rich regions, which exhibit smoother surfaces without branching. This is again in stark contrast to the Sn-rich globules that form in the case of the Sn-Bi alloy. Notably, while the Sn-40Bi-10In alloy does not qualify as a eutectic alloy, the growth of a Sn/Bi/BiIn ternary was detected in the interglobular areas, as shown in Fig. 4 for the sample treated for 12 h at 120°C. An increasing fraction of dendrites develop in concurrence with this ternary eutectic, as will be seen later in the section “Evolution of Interglobular Eutectic Microstructure.” Some of the EDS spectra in Fig. 4 confirm the formed phases, i.e., β-Sn, Bi, and BiIn.
Evolution of Sn-Rich Globule Morphology
The increase in the size of the Sn-rich globules is a diffusion-driven process powered by capillary forces. According to Marsh and Glicksman,23 capillary-driven diffusion depends on the dispersion of interfacial curvatures throughout a complex structure in the alloy systems, which have typical solid-meld microstructural size scales. On a typical interface, the solute concentration varies locally due to variations in mean curvature. These resulting concentration gradients contribute to the phenomenon of coarsening of the globules. Therefore, monitoring variations in local compositions with geometric alterations is crucial.24 This type of microstructure significantly reduces the apparent viscosity, enhancing the flow of semisolid material.
Figure 5 shows the evolution of globule size (equivalent diameter) and area fraction as a function of aging time. It is evident that the equivalent diameter and the globule area fraction increase with time in the Sn-Bi-In alloy samples. This indicates that at extended durations (360 h), a reduced fraction of liquid is present in the interglobular regions. Notably, after 12 h of heat treatment, the average globule diameter in the Sn-40Bi alloy is nearly twice as large as that in the Sn-Bi-In alloy. The variation in globule size when comparing the two alloys primarily stems from distinctions in the initial microstructures (see Fig. 3). In Sn-Bi-In samples, the lack of dendritic structures at the globule interface seems to promote the development of these globules. This is particularly noticeable in the rounded Sn-rich regions in the untreated Sn-Bi-In samples (Fig. 3a), where smoother surfaces without branching are observed, resulting in finer globules.
Evolution of the globule morphology and area fraction as a function of heat treatment time: The globule size in Sn-40Bi at 12-h treatment time is twice that of Sn-40Bi-10In at the same treatment time, and further heat treatment of the latter composition slows the significant development of globule size while the area fraction of the globules increases more conservatively.
Evolution of Intra-globular Microstructure
Figure 6 shows the Z-contrast images of regions within the globules, highlighting the changes that occur with heat treatment. Z-contrast is an SEM imaging mode sensitive to the atomic weight of the elements present. Since Bi is the heaviest material within the alloys, regions rich in Bi are seen as bright regions or spots (indicated by yellow arrows) in the Z-contrast images. The as-soldered samples (Fig. 6a and e) solidify slowly during cooling, resulting in the formation of coarser precipitates. The extended cooling period allows Bi sufficient time to diffuse towards precipitation sites, promoting coarsening.
The 12-h-treated Sn-Bi-In sample, shown in Fig. 6b, with a slower cooling rate, led to a different precipitation pattern than the fast-cooled 168-h and 360-h samples. This discrepancy is attributed to the reduced remaining liquid content in the latter samples before undergoing water quenching. Samples subjected to shorter treatment periods exhibited reduced fractions of Sn-rich globules, as depicted in Fig. 5. Consequently, before quenching, a larger amount of liquid remained with a higher accumulated energy, when compared to longer treatment periods, leading to a slower cooling process. Moreover, prolonged aging time permits Bi to diffuse towards multiple precipitation sites, facilitating a more even distribution forming a mixture of Bi globules and plates. In contrast, the 360-h sample (Fig. 6d) undergoes rapid cooling, impeding Bi diffusion while it remains in a supersaturated state. This limits the time available for Bi to diffuse and redistribute, resulting in a higher precipitation rate and lower diffusion. Some regions of this sample remain locked and unprecipitated, while larger particles grow at the expense of smaller ones in other areas.
The Bi precipitates formed after the heat treatment of the Sn-40Bi alloy for 12 h are considerably finer and feature a mixture of globules and plates. In contrast, the untreated Sn-Bi sample exhibited regions with elongated plates, resulting from facilitated diffusion in the absence of In (Fig. 6e). When comparing the fractions of globules formed for the Sn-Bi and Sn-Bi-In alloys for 12 h in Fig. 5, the results are 74.9% and 55.6%, respectively. This implies that the cooling rate in the quenching step for the Sn-40Bi alloy was higher, as the remaining liquid was lower.
The 12-h-treated Sn-40Bi alloy (Fig. 6f) exhibits significantly smaller Bi particles, indicating a more pronounced refining effect than the Sn-Bi-In alloy under identical treatment conditions. A bimodal-type distribution of particles characterizes the 12-h Sn-Bi sample. Solute segregation appears to occur within the β-Sn globules, resulting in areas with higher Bi concentration, which act as more abundant nucleation sites during the precipitation stage. Conversely, regions with lower Bi content are observed, and this alternates across the globules.
Evolution of Intra-globular Composition
The EDS data from the intra-globular regions shown in Fig. 6 indicate that Bi is absorbed into the β-Sn matrix during the semisolid treatment via the globular/liquid interface. This is evident from the increase in Bi seen in the data for Sn-Bi-In in Fig. 7a. The Bi content (at.%) increased from 12.9 (0 h) to 15.6 (360 h). The In content remained nearly constant over time. The supersaturated Bi precipitates upon cooling to room temperature. This compositional variation impacts microstructural stability across the globules.
Figure 7b shows the Bi particle size, circularity, and count density values for all the analyzed samples. Circularity here is a dimensionless quantity (from 0 to 1) to numerically describe the shape of a particle, independent of its size. A steady coarsening of larger Bi particles occurred over the as-reflowed sample shown in Fig. 6a, resulting in a lower circularity and a higher average area than other Sn-Bi-In samples. The reduced Bi content within the β-Sn matrix could justify the lower area fraction observed in this as-reflowed sample. The 12-h sample cools slowly and with high Bi in the solution. In this case, Bi has time to diffuse towards more precipitation sites during the cooling period, favoring better distribution of the particles. An area fraction 50% higher in Fig. 7b was observed compared to the as-soldered samples. The 360-h sample exhibited an average Bi particle area that was half the size of the 12-h sample. However, circularity and area fraction remained relatively consistent when comparing data from the 12-h and 360-h samples.
When comparing the 12-h treatment data of the two alloys (with and without In in Fig. 7c), it is observable that nearly the same average Bi concentration is obtained (14.4 at.% for Sn-40Bi versus 14.8 at.% for Sn-40Bi-10In) for the regions presented in Fig. 6. The samples without In exhibit a significantly smaller average Bi particle area but a higher area fraction of Bi. This may be explained by the fact that In appears to decrease the precipitation capacity, reducing the number of viable sites in the supersaturated state. In hinders the mobility of Bi in solution, retaining atoms of this element in the Sn-rich matrix, which do not become nucleation sites. The diffusion coefficient of In in Sn is lower than that of Bi in Sn, with both elements having similar atomic radii.25,26,27 These characteristics ultimately decrease the mobility of Bi in the Sn crystalline lattice. Furthermore, by examining Bi in β-Sn solution after the cooling process (see Fig. 8 and Table II), an increased residual amount of Bi is observable within the Sn-Bi-In samples that underwent treatment. This observation indicates that the presence of In reduces the number of nucleation sites, consequently affecting the nucleation process.
Evolution of Interglobular Eutectic Microstructure
The evolution of the interglobular eutectic microstructure as a function of semisolid treatment is shown in Fig. 9 through SEM Z-contrast images. The Sn phase appears in black, the BiIn phase in gray, and the Bi phase in white. A simple grayscale-based segmentation of the three phases was carried out using Avizo software and is presented in the adjoining false-color images in Fig 9. A ternary eutectic in this alloy is rather unexpected and can only be attributed to composition fluctuations over an extended time. The transfer of Bi from the interglobular liquid to the Sn-rich matrix over the treatment period allowed a new proportion to develop between the elements in the liquid zone, approaching the eutectic composition. It is observable that over time, the formation of β-Sn dendritic structures increased, with a higher fraction observed for 360 h.
Using a systematic investigation of solders with compositions spanning the valley line between the Sn-Bi eutectic and the ternary β-Sn/Bi/BiIn eutectic point at 76°C27 (as depicted in Fig. 10) in addition to EDS-based compositional analysis of the interglobular regions (shown in Fig. 9), the three compositions for samples heat-treated for 12 h, 168 h, and 360 h are charted in the ternary phase diagram shown in Fig. 10. In the case of the 12-h sample, the predominant constituent was primarily the eutectic Sn + Bi, leaning towards the formation of the ternary eutectic due to non-equilibrium solidification. However, for the 168-h and 360-h samples, the primary constituent consisted of β-Sn followed by the eutectic reaction.
For the interglobular eutectic region, the phase fractions shown in Fig. 11a correlate well with the EDS results in Fig. 11b. The 360-h sample exhibits a higher Sn content and lower Bi content in the interglobular region compared to the 12-h sample. This enables the growth of a larger fraction of dendrites in the interglobular areas in the 168-h and 360-h samples.
Figure 12 shows the morphological parameters such as Feret shape and aspect ratio for the Bi and BiIn phases. No notable differences were observed among the 12-h, 168-h, and 360-h samples. However, the size of the phases, both Bi and BiIn, tend to increase with prolonged heat treatment times. This can be attributed to the varying solidification paths during the cooling process, as shown in Fig. 10. The initial formation of the β-Sn primary phase involves the release of sensible heat until the ternary eutectic composition is achieved, enabling this reaction. This portion of heat decreases the eutectic growth rate during cooling after the 168-h and 360-h treatments. As a result, it forms a coarser eutectic structure with a larger average area and particle length for the Bi and BiIn, especially in the samples heat-treated for a 360-h period.
Conclusions
The changes in the microstructure as a function of long-term heat treatment for Sn-40Bi and Sn-40Bi-10In alloys were reported. The evolution of inter- and intra-globular microstructures was studied using CALPHAD, SEM imaging, EDS, and quantitative image analysis. The following conclusions can be drawn from this study:
-
(1)
Sn-40Bi-10In alloy consisted of Sn-rich islands in an as-reflowed state, while the Sn-40Bi alloy had Sn-rich dendrites. The absence of dendritic structures promoted the development of finer globules in the Sn-Bi-In samples. After undergoing semisolid aging, the Sn-Bi-In alloy formed Sn-rich globules containing In and Bi, surrounded by fine ternary eutectic zones.
-
(2)
The coarsening of Sn-rich globules over time was driven by diffusion and capillary forces and influenced by variations in solute content due to mean curvature changes at interfaces. An increased equivalent diameter and globule area fraction were observed with aging time in Sn-Bi-In alloy samples. This suggested a reduced liquid fraction in interglobular regions over an extended aging time (360 h).
-
(3)
Slow cooling of the as-soldered samples led to coarser precipitates due to prolonged Bi diffusion. In contrast, the 360-h Sn-Bi-In sample underwent rapid cooling, limiting Bi diffusion and resulting in locked regions with larger particles. The 12-h Sn-40Bi sample exhibited a bimodal distribution of particles, indicating solute segregation and varied nucleation sites.
-
(4)
During semisolid treatment, EDS revealed Bi absorption into the β-Sn matrix through the globular/liquid interface. The supersaturated Bi precipitated upon cooling, impacting microstructural stability. The 12-h Sn-Bi-In sample exhibited a higher precipitate Bi area fraction than the untreated sample. The presence of In reduced the number of viable sites for nucleation and affected the nucleation process.
-
(5)
The unexpected ternary Sn/Bi/BiIn eutectic formation suggested that liquid composition changed over treatment time. Longer treatment times allowed a higher fraction of β-Sn dendritic structures within the interglobular zones. Morphological parameters at this zone indicated increased Bi and BiIn phases with prolonged heat treatment, attributed to varying solidification paths.
Data Availability
Data presented in this study are available on request from the corresponding author.
References
R.S. Sidhu, M.P. Renavikar, A.A. Dani, M.A. Dudek, Solder paste material technology for elimination of high warpage surface mount assembly defects. U.S. Patent, 0175160 A1 (2014)
K.J. Puttlitz and K.A. Stalter, High-temperature lead-free solders with dispersoids, Handbook of Lead-Free Solder Technology for Microelectronic. ed. K.J. Puttlitz, and K.A. Stalter (New York: Marcel Dekker, 2004).
W. Zhang, J. Zhao, Z. Yin, N. Zhou, L. Tang, and C. Suo, Study on wettability of low-melting alloy material on copper substrates. Ferroelectrics 549, 160–171 (2019).
C.K. Roy, S. Bhavnani, M. Hamilton, W.R. Johnson, R.W. Knight, D.K. Harris, Performance of Low Melt Alloys as Thermal Interface Materials, in IEEE 31st SEMI-THERM Symposium (2015)
X. Wu, J. Wu, X. Wang, J. Yang, M. Xia, and B. Liu, Effect of In addition on microstructure and mechanical properties of Sn–40Bi alloys. J. Mater. Sci. 55, 3092–3106 (2020).
G. Siroky, E. Kraker, J. Rosc, D. Kieslinger, R. Brunner, S. van der Zwaag, E. Kozeschnik, and W. Ecker, Analysis of Sn-Bi solders: X-ray micro computed tomography imaging and microstructure characterization in relation to properties and liquid phase healing potential. Materials 14, 153 (2021).
C. Fisher, H. Henderson, M. Kesler, P. Zhu, G. Bean, and M. Wright, Repairing large cracks and reversing fatigue damage in structural metals. Appl. Mater. Today 13, 64–68 (2018).
G. Siroky, E. Kraker, J. Magnien, D. Melinc, D. Kieslinger, E. Kozeschnik, and W. Ecker, Effect of solder joint size and composition on liquid-assisted healing. Microelectron. Reliab. 119, 114066 (2021).
Y. Liu and K. Tu, Low melting point solders based on Sn, Bi, and In elements. Mater. Today Adv. 8, 100115 (2020).
Q. Li, N. Ma, and Y. Lei, Characterization of low-melting-point Sn–Bi–In lead-free solders. J. Electron. Mater. 45, 5800–5810 (2016).
R. Shalaby, Effect of silver and indium addition on mechanical properties and indentation creep behavior of rapidly solidified Bi–Sn based lead-free solder alloys. Mater. Sci. Eng. A 560, 86–95 (2013).
I.E. Anderson, J.C. Foley, and B.A. Cook, Alloying effects in near-eutectic Sn–Ag–Cu solder alloys for improved microstructural stability. J. Electron. Mater. 30, 1050–1059 (2001).
T. Ventura, Y. Cho, C. Kong et al., Formation of intermetallics in Sn–0.9Cu and Sn–0.7Cu–0.08Ni solders. J. Electron. Mater. 40, 1403–1408 (2011).
C. Yu, W. Chen, and J. Duh, Suppressing the growth of Cu–Sn intermetallic compounds in Ni/Sn–Ag–Cu/Cu–Zn solder joints during thermal aging. Intermetallics 26, 11–17 (2012).
K.R. Hassan, J. Wu, M.S. Alam, J.C. Suhling, and P. Lall, Mechanical behavior and reliability of SAC+Bi lead-free solders with various levels of bismuth, in 2021 IEEE 71st Electronic Components And Technology Conference (ECTC) (IEEE, 2021), pp. 937
A.A. El-Daly, A.M. El-Taher, and S. Gouda, Novel Bi-containing Sn–1.5Ag–0.7Cu lead-free solder alloy with further enhanced thermal property and strength for mobile products. Mater. Des. 65, 796–805 (2015).
S.A. Belyakov, J. Xian, G. Zeng, K. Sweatman, T. Nishimura, T. Akaiwa, and C.M. Gourlay, Precipitation and coarsening of bismuth plates in Sn–Ag–Cu–Bi and Sn–Cu–Ni–Bi solder joints. J. Mater. Sci. Mater. Electron. 30, 378–390 (2019).
J.A. Wu, A. Luktuke, and N. Chawla, Surface precipitation and growth of bismuth particles in Sn–Ag–Cu–Bi solder joints. J. Electron. Mater. 52, 801–809 (2023).
F. Wang, A. Luktuke, and N. Chawla, Microstructural coarsening and mechanical properties of eutectic Sn-58Bi solder joint during aging. J. Electron. Mater. 50, 6607 (2021).
P.T. Vianco and J.A. Rejent, Properties of ternary Sn–Ag–Bi solder alloys: part I - thermal properties and microstructural analysis. J. Electron. Mater. 28, 1127–1137 (1999).
M. H. Biglari, A. Das, L.C.P. Krassenburg, J.H.G. Brom, N.J.A. van Veen, A. A. Kodentsov, Low-temperature soldering using ordered alloys, in Proceedings of SMTA International, Oct. 14–18, Rosemont, IL, USA (2018)
M.C. Flemings, Behavior of metal alloys in the semisolid state. Metall. Trans. A 22, 957–981 (1991).
S.P. Marsh and M.E. Glicksman, Overview of geometric effects on coarsening of mushy zones. Metall. and Mater. Trans. A. 27, 557–567 (1996).
O. Lashkari, R Ghomashchi The implication of rheology in semi-solid metal processes: an overview. J. Mater. Process. Technol. 182, 229–240 (2007).
A. Sawatzky, Diffusion of indium in tin single crystals. J. Appl. Phys. 29, 1303–1305 (1958).
A.M. Delhaise, Solid-State Diffusion of Bismuth in Tin-Rich. Lead-Free Solder Alloys (Toronto: University of Toronto, 2018).
S. Tian, S. Li, and J. Zhou, Effect of indium addition on interfacial IMC growth and bending properties of eutectic Sn–07.Cu solder joints. J. Mater. Sci. Mater. Electron. 28, 16120 (2017).
S.R. Mang, H. Choi, and H.J. Lee, Investigation of Sn–Bi–In ternary solders with compositions varying from Sn–Bi eutectic point to 76°C ternary eutectic. J. Mater. Sci. Mater. Electron. 33, 17453–17461 (2022).
Acknowledgments
The authors acknowledge FAPESP (Grants 2019/23673-7, 2021/08436-9, and 2023/06107-3) and CNPq. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) - Finance Code 001. JAW, EG, AL, and NC acknowledge financial support from the U.S. Partnership for Assured Electronics (USPAE) on the project “Lead-Free Solder Performance and Reliability Assurance.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spinelli, J.E., Leal, J.R., Wu, J.A. et al. Long-Term Microstructural Stability of Sn-40Bi and Sn-40Bi-10In Alloys. J. Electron. Mater. 53, 2455–2466 (2024). https://doi.org/10.1007/s11664-024-10995-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-024-10995-0