In a significant stride towards enhancing chemical safety and environmental protection, Brazil is in the process of establishing its own regulatory framework for the management of chemicals, often referred to as "Brazil REACH." At the forefront of this development is Bill PL 6120/2019, a legislative proposal that is set to transform the chemical industry landscape in Brazil.
Introduction to Bill PL 6120/2019
The Brazilian Chamber of Deputies' Commission on Economic Development, Industry, Trade, and Commercial Services (CDEICS) took a pivotal step on November 23, 2022, when it passed Bill PL 6120/2019. This landmark legislation, once approved, will serve as the cornerstone of the Brazilian REACH Regulation, ushering in a new era of chemical management and safety.
A National Inventory of Chemicals
One of the fundamental aspects of Bill 6120/2019 is the creation of a national inventory of chemicals. This inventory will encompass a wide array of chemical substances produced, consumed, or stored in Brazil. From this vast inventory, a group of high-priority chemicals will be selected for rigorous risk evaluations. Simultaneously, substances that necessitate authorization will be identified and regulated. This comprehensive inventory is a crucial step toward ensuring transparency and accountability in the chemical industry.
Joining the Ranks of South American Nations
If Bill 6120/2019 successfully passes into law, Brazil will join the ranks of South American nations that have already established regulations for chemical management. Currently, Chile and Colombia stand as the pioneers in this regard. Brazil's entry into this exclusive group signifies its commitment to aligning its standards with international practices and enhancing chemical safety within its borders.
Substance Declaration: Who Needs to Declare?
Bill PL 6120/2019 sets clear guidelines on which entities need to declare the presence of chemical substances. In essence, chemical substances produced, consumed, or stored in Brazil in quantities exceeding one ton per year (t/a) over the last three years must make declarations. This requirement ensures that a comprehensive chemical inventory is compiled, providing valuable insights into the chemical landscape of the country.
Key Features of Bill 6120/2019
-
Manufacturer's and Importer's Identity Information: The bill mandates the provision of identity information for manufacturers and importers, as specified by legislation.
-
Precise Naming of Chemical Compounds: This includes the use of names and CAS numbers assigned by recognized bodies like the Chemical Abstracts Service (CAS) or the International Union of Pure and Applied Chemistry (IUPAC).
-
Chemical Substance Production and Import Statistics: Accurate statistics related to the production and import of chemical substances are required.
-
Suggested Uses for Chemicals: The bill calls for clear documentation of suggested uses for chemical substances.
-
GHS and Brazilian Regulatory Hazard Classifications: The bill encompasses the classification of chemicals in accordance with the Globally Harmonized System (GHS) and Brazilian regulatory hazard classifications.
Exemptions under Bill PL 6120/2019
It is important to note that Bill 6120/2019 exempts certain substances from its provisions. These exemptions include:
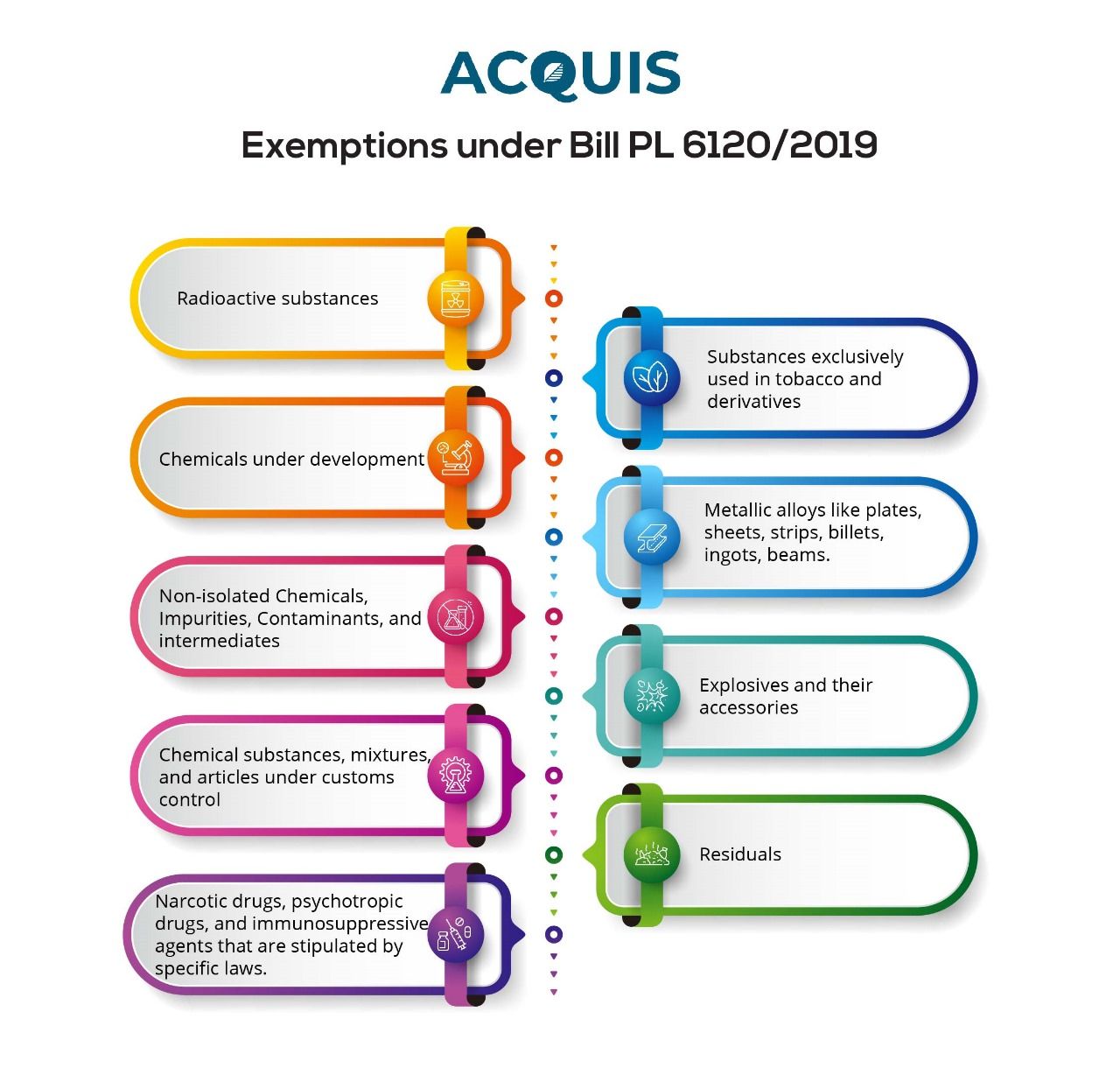
- Radioactive substances
- Chemicals under development
- Non-isolated Chemicals, Impurities, Contaminants, and intermediates
- Chemical substances, mixtures, and articles under customs control
- Narcotic drugs, psychotropic drugs, and immunosuppressive agents that are stipulated by specific laws.
- Substances exclusively used in tobacco and derivatives
- Metallic alloys like plates, sheets, strips, billets, ingots, beams.
- Explosives and their accessories
- Residuals
Products that are regulated by specific laws and regulations:
- Pesticides and related products, premix, and technique products;
- Pharmaceuticals and medical gases;
- Cosmetics, toiletries, and perfumes;
- Disinfectants;
- Products for veterinary uses;
- Manufacturing technologies of food, food additives, and accessories;
- Products used for animal feed;
- Fertilizers, inoculants, and correctives;
- Wood preservatives; and
- Environmental restoration agents.
Chemically modified or are composed of or contain substances harmful to health or the environment according to GHS.
- Ores and their concentrates, and other rocks and minerals, including components for manufacturing coal, coke, crude oil, natural gas, liquefied natural gas, natural gas condensate, gas, and mineral; Natural substances.
- Fats, essence oils, and fixed oils are extracted by the method of grinding, pressing, or bleeding, even if purified if they yield a product with the same characteristics as the original product glass and ceramics.
Registration of New Substances
Once the national existing chemical substance inventory is finalized, any chemical substances not listed will be considered as new chemical substances. Manufacturers and importers dealing with quantities equal to or exceeding one tonne per year (>=1t/y) of new chemical substances are required to register these substances. Registration involves submitting additional studies and a risk assessment report. The data requirements for registration will increase with different tonnage bands, ensuring a more rigorous evaluation for higher-risk substances.
Criteria for the Selection of New Chemical Substances
Bill 6120/2019 outlines specific criteria for the selection of new chemical substances. These criteria include:
- Bioaccumulation in the environment and persistence
- Contamination of the environment
- Reproductive toxicity, carcinogenicity, and mutagenicity
- Qualities of an endocrine disruptor based on scientific research
- Risks comparable to exposure to people and the environment
- Substances covered by an international alert or governed by a treaty, convention, or agreement to which Brazil is a party.
Concerned Parties and Compliance
Bill 6120/2019 places significant responsibilities on manufacturers, producers, and importers of industrial chemical substances. Those dealing with one tonne or more of chemical substances annually are required to report the quantity of substances produced and imported each year. They must also provide Safety Data Sheets (SDSs) in accordance with the Globally Harmonized System (GHS), which includes information about recommended uses, hazard classifications, and chemical risks.
Foreign manufacturers and formulators also have the option to designate an Only Representative for compliance-related reasons. This allows them to navigate the regulatory landscape in Brazil effectively.
Compliance with Brazilian Regulatory Standards
Manufacturers in Brazil are bound by the Brazilian Regulatory Standards NR9 and NR15, which provide instructions on necessary procedures related to occupational safety and health. Standard NR15 establishes tolerance levels for chemical agents in the workplace, while Standard NR9 outlines the requirements for enterprises to have an environmental risk prevention program. These standards ensure that safety and environmental protection are paramount in the chemical industry.
Registration and Authorization Requirements
Both companies and individuals in Brazil are required to adhere to the Brazilian Federal Police's registration and authorization requirements, as well as transportation laws. If a material is mentioned in Annex I of Ordinance No. 204 of October 21, 2022, companies must register with the Federal Police to obtain the following documentation:
-
Cadastral Registration Certificate (CRC): This certificate attests to the proper registration of a natural person or legal entity with the Federal Police.
-
Operating Licence Certificate (CLF): This certificate demonstrates the eligibility of a legal entity to engage in activities involving chemical products.
-
Special Authorization (AE): This document certifies the right of a natural person or legal entity to conduct future operations involving chemical products.
Registration Certificate and an Operating License Certificate (CLF) must be possessed by all parties involved, including traders, manufacturers, and carrying bodies. The CLF must be renewed annually, ensuring continued compliance with regulatory standards. In exceptional cases, a Special Authorization may be issued, contingent upon factors such as the approval of registration, the applicant's economic activity, and the intended future use of the product.
Conclusion: Preparing for Change
In conclusion, Bill PL 6120/2019 represents Brazil's effort to establish its own chemical regulatory framework, often referred to as "Brazil REACH." If enacted into law, this regulation will bring significant changes to the chemical industry in Brazil, aiming to enhance chemical safety and environmental protection while aligning with international standards. Companies operating in this sector should prepare for compliance with the new regulatory framework.
Speak to Our Compliance Experts
Share
ENVIRONMENTAL COMPLIANCE
- RoHS
- SCIP (WFD)
- REACH
- California Proposition 65
- Material Disclosure (FMD)
- PFAS
- TSCA PBT
- EU POPs
- EU MDR & IVDR
- ELV (GADSL)
- Others
- Extended Producer Responsibility (EPR)
INTEGRATION SUPPORT
WE ARE GLOBAL
USA
6705 Ridgedale CT, Glen Allen, VA 23059
+1.757.801.2760
info@aquiscompliance.com
India
#9/2, Hennur Bagalur Main Road, Bengaluru - 560077
+91 789 238 1827
info@aquiscompliance.com



