- 1Univ Lyon, CarMeN Laboratory, INSERM, INRA, INSA Lyon, Université Claude Bernard Lyon 1, Bron, France
- 2Olea Medical, La Ciotat, France
- 3Hospices Civils de Lyon, Hôpital Louis Pradel, Services D’explorations Fonctionnelles Cardiovasculaires et CIC de Lyon, Lyon, France
Background: The immune system, composed of organs, tissues, cells, and proteins, is the key to protecting the body from external biological attacks and inflammation. The latter occurs in several pathologies, such as cancers, type 1 diabetes, and human immunodeficiency virus infection. Immunophenotyping by flow cytometry is the method of choice for diagnosing these pathologies. Under inflammatory conditions, the peripheral blood mononuclear cells (PBMCs) are partially activated and generate intracellular pathways involving Ca2+-dependent signaling cascades leading to transcription factor expression. Ca2+ signaling is typically studied by microscopy in cell lines but can present some limitations to explore human PBMCs, where flow cytometry can be a good alternative.
Objective: In this review, we dived into the research field of inflammation and Ca2+ signaling in PBMCs. We aimed to investigate the structure and evolution of this field in a physio-pathological context, and then we focused our review on flow cytometry analysis of Ca2+ fluxes in PBMCs.
Methods: From 1984 to 2022, 3865 articles on inflammation and Ca2+ signaling in PBMCs were published, according to The Clarivate Web of Science (WOS) database used in this review. A bibliometric study was designed for this collection and consisted of a co-citation and bibliographic coupling analysis.
Results: The co-citation analysis was performed on 133 articles: 4 clusters highlighted the global context of Ca2+ homeostasis, including chemical probe development, identification of the leading players in Ca2+ signaling, and the link with chemokine production in immune cell function. Next, the bibliographic coupling analysis combined 998 articles in 8 clusters. This analysis outlined the mechanisms of PBMC activation, from signal integration to cellular response. Further explorations of the bibliographic coupling network, focusing on flow cytometry, revealed 21 articles measuring cytosolic Ca2+ in PBMCs, with only 5 since 2016. This final query showed that Ca2+ signaling analysis in human PBMCs using flow cytometry is still underdeveloped and investigates mainly the cytosolic Ca2+ compartment.
Conclusion: Our review uncovers remaining knowledge gaps of intracellular players involved in Ca2+ signaling in PBMCs, such as reticulum and mitochondria, and presents flow cytometry as a solid option to supplement gold-standard microscopy studies.
1 Introduction
Blood samples are routinely used in clinics for disease diagnosis or prognosis. The main components of blood are plasma and leukocytes. The immunophenotyping of leukocytes, notably the peripheral blood mononuclear cells (PBMCs), has emerged as an essential tool for medical research. This diagnostic tool is widespread in hematology, cancerology, and neurology fields.
For clinical diagnosis, multiparametric phenotyping is typically achieved using flow cytometry. This method achieves 10000 to 40000 cell reads per second on average with the most recent cytometers, enabling statistical robustness compared to microscopy imaging, high sensitivity and specificity to detect the most under-represented subpopulations, and fast diagnosis (usually under 48 hours) (1, 2). Diagnosis is based on detecting PBMC-specific membrane glycoproteins called “cluster of differentiation” (CD), commonly associated with their immune function and cell subpopulation (3). The simultaneous expression of multiple CD markers serves as a cellular signature comparable to an “identity card.” PBMCs can easily be isolated from blood sampling followed by centrifugation. Therefore, PBMCs have recently emerged as complementary biomarkers to stratify patients in several pathologies by characterizing their immunophenotypes. For instance, monitoring the level of CD4+ T cells in Human Immunodeficiency Virus (HIV) infections is crucial to monitoring disease progression (4). Flow cytometry has also become essential to study the activation of basophilic cells in the presence of a given allergen (5) and can even be used to diagnose acute leukemia (6). In fact, flow cytometry is extensively used in several clinical applications notably in clinical practice. Indeed, flow cytometry is largely used to characterize diseases such as malignancies (leukemia, lymphoma (7, 8)), infectious diseases (9) and degenerative diseases (10, 11) through immunophenotyping. More recently, Obasanmi et al. studied PBMC cytokine production levels in patients with type 1 diabetes and diabetic retinopathy. Their work revealed that PBMCs from diabetic patients have a specifically enhanced interleukin-10 (IL-10) and interleukin-6 (IL-6) releases, associated with increased interleukin-17A (IL-17A) production from myeloid cells and impaired CD3+ T cell-induced interferon-gamma (IFN-γ) production (12). Flow cytometry is recognized as the method of choice for immunophenotyping based on this non-exhaustive list of examples.
Under inflammatory conditions, PBMCs are partially activated by T or B cell membrane receptors (TCR or BCR, respectively) and Fc receptors for monocytes and macrophages. Their activation through these receptors involves a Ca2+-dependent signaling cascade, starting from the ligand binding on the receptor through intracellular pathways up to the regulation of gene expression. More precisely, membrane receptor activation triggers a transitory Ca2+ release from internal stores, further leading to a store-operated Ca2+ entry (SOCE), raising the intracellular Ca2+ concentration (13, 14), which in turn, regulates several processes such as proliferation, phagocytosis, chemotaxis, and cytokine secretion (15). Thus, Ca2+ is considered as a key regulator of the immune cell. In 1994, Partiseti et al. reported an altered Ca2+ influx following TCR aggregation in native T cells in immunodeficient patients, visualized with microscopy and electrophysiological recordings (16). So far, microscopy is the most common method to identify and analyze Ca2+ fluxes in cell line models. It offers the advantage of visualizing the intracellular architecture of the cell. However, the statistical power brought by the number of events analyzed, the high acquisition rate, and the possibility of exploring a more significant number of parameters simultaneously on the same sample all favor the use of flow cytometry. Consequently, it represents a powerful alternative to assess Ca2+ fluxes, but still remains underused. It has been shown that alteration or modulation of calcium signaling can impact immune cell function in some pathologies (17, 18). To identify existing protocols for analyzing calcium signaling, we proposed this bibliometric review questioning the role of flow cytometry in studying Ca2+ signaling, specifically in peripheral blood mononuclear cells. To this end, we first outlined the inflammatory and Ca2+ research field and then focused on Ca2+ homeostasis studies performed by flow cytometry on PBMCs.
2 Materials and methods
2.1 Bibliometric analysis
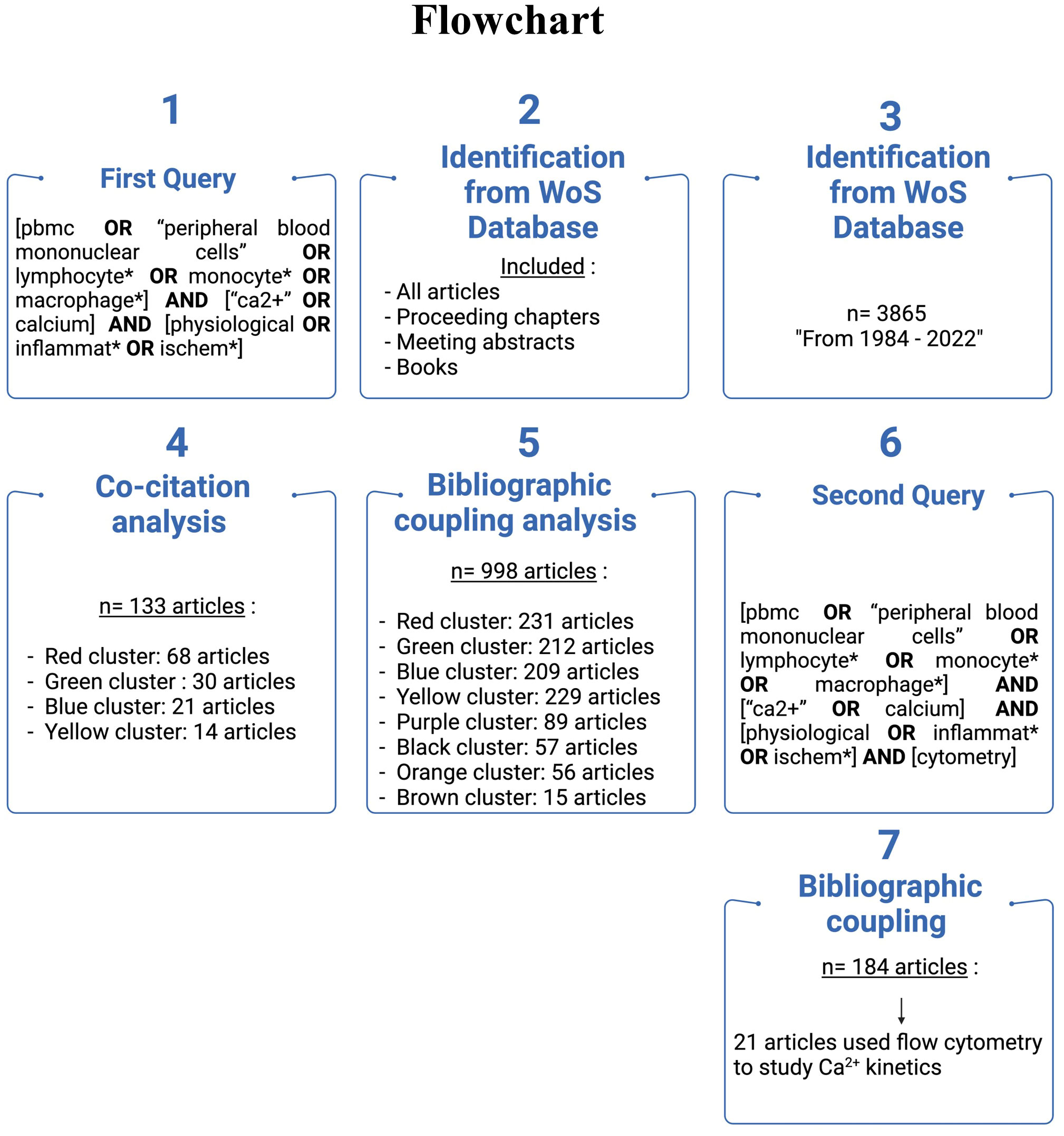
The bibliometric analysis is suited when the scope of the review is broad and the dataset is too large for manual review. This analysis presents the intellectual structure and emerging trends of a research topic. The study was designed following the guidelines of Donthu et al. and the methodology of Chalet et al. (19, 20). The flowchart depicted in Figure 1 summarizes the methodology used to highlight the literature selection.
2.2 Scope and aim of the analysis
We aimed to determine the research structure and evolution of inflammatory and Ca2+ signaling in PBMCs in a physio-pathological context. Based on these first results, we further explored the development of Ca2+ analysis by flow cytometry.
2.3 Definition and selection of bibliometric analysis techniques
The structure and dynamics of the scholar knowledge were assessed and represented using science maps. To provide an overview of our research topic and to explore the emerging trends, co-citation analysis and bibliographic coupling were carried out.
- The co-citation analysis determines the relationships among cited publications, highlighting the most influential themes.
- The bibliographic coupling forms thematic clusters.
2.4 Collecting scientific literature data
Clarivate Web of Science© was used to collect data (Copyright Clarivate 2022WoS). It is a selective, structured, and balanced database with complete citation links and enhanced metadata. It can inform about citation indexes representing the connections between scholarly research articles in globally significant journals, books, and proceedings chapters. Exported data included the complete set of references enabling in-depth analysis of the intellectual structure.
We applied the following query to article titles and abstracts:
[pbmc OR “peripheral blood mononuclear cells” OR lymphocyte OR monocyte* OR macrophage*] AND [“Ca2+” OR calcium] AND [physiological OR inflammat* OR ischem*]
We included all articles, proceeding chapters, meeting abstracts, and books. The query ranged from 1984 to 2022, and 3865 articles were collected. All data were exported from the WoS database on July 22, 2022.
Further analysis of the field, focusing on the evolution of Ca2+ analysis by flow cytometry, was carried out based on the following query applied to abstracts and titles:
[pbmc OR “peripheral blood mononuclear cells” OR lymphocyte* OR monocyte* OR macrophage*] AND [“Ca2+” OR calcium] AND [physiological OR inflammat* OR ischem*] AND [cytometry]
We included all articles, book chapters, and meeting abstracts. The query ranged from 1993 to 2022, and 185 articles were obtained.
2.5 Network generation and display
VOS viewer was used to display bibliometric networks represented through mapping and clustering (21) to perform the analysis.
2.6 Co-citation method
Two articles are co-cited when they appear together in the reference list of another publication. Frequently co-cited documents in a corpus represent its knowledge foundations. This technique highlights influential publications and unveils the structure of the research field. The co-citation was conducted in VOS viewer using reference analysis. The results were displayed as network visualization. The minimum number of co-cited references was fixed to 20 to obtain the most influential articles. 133 co-cited documents were included in our analysis and mapping, and were clustered and displayed on the VOS viewer. Included references grouped by clusters are displayed in Supplementary Figure S1. To determine the main subject of each cluster, we focused our analysis on the most significant publications in each cluster, i.e., in the 3rd quartile.
2.7 Bibliographic coupling method
The bibliographic coupling method considers publications that share common references as an indication of similarities in content. This analysis provides a clustered visualization of the field in themes and includes recent and niche publications. The bibliographic coupling analysis was obtained with VOS viewer, with coupling analysis of references. The results were displayed as network visualization. This bibliographic coupling was carried out to determine the knowledge in the field of Ca2+ and inflammation in PBMCs. Articles from 1990 to 2022 were included, and 998 were grouped in clusters. Cluster constituents are detailed in Supplementary Figure S2.
The exploration of the clusters was performed on the highest total link strength articles, corresponding to the link of an item with other items in a network. To get an overview of the dominant theme of each cluster, the references with a total link strength equal to or higher than the 3rd quartile of the full scores in their cluster were selected for a thorough analysis.
2.8 Limitations of bibliographic coupling analysis
The term “inflammat*” covers a large field of research, including several pathologies. Thus, the most influential articles in our “inflammatory” cluster discuss chemokines in HIV. The abbreviation T cells or B cells were not included in the query.
3 Results
3.1 General information: Trend of publication
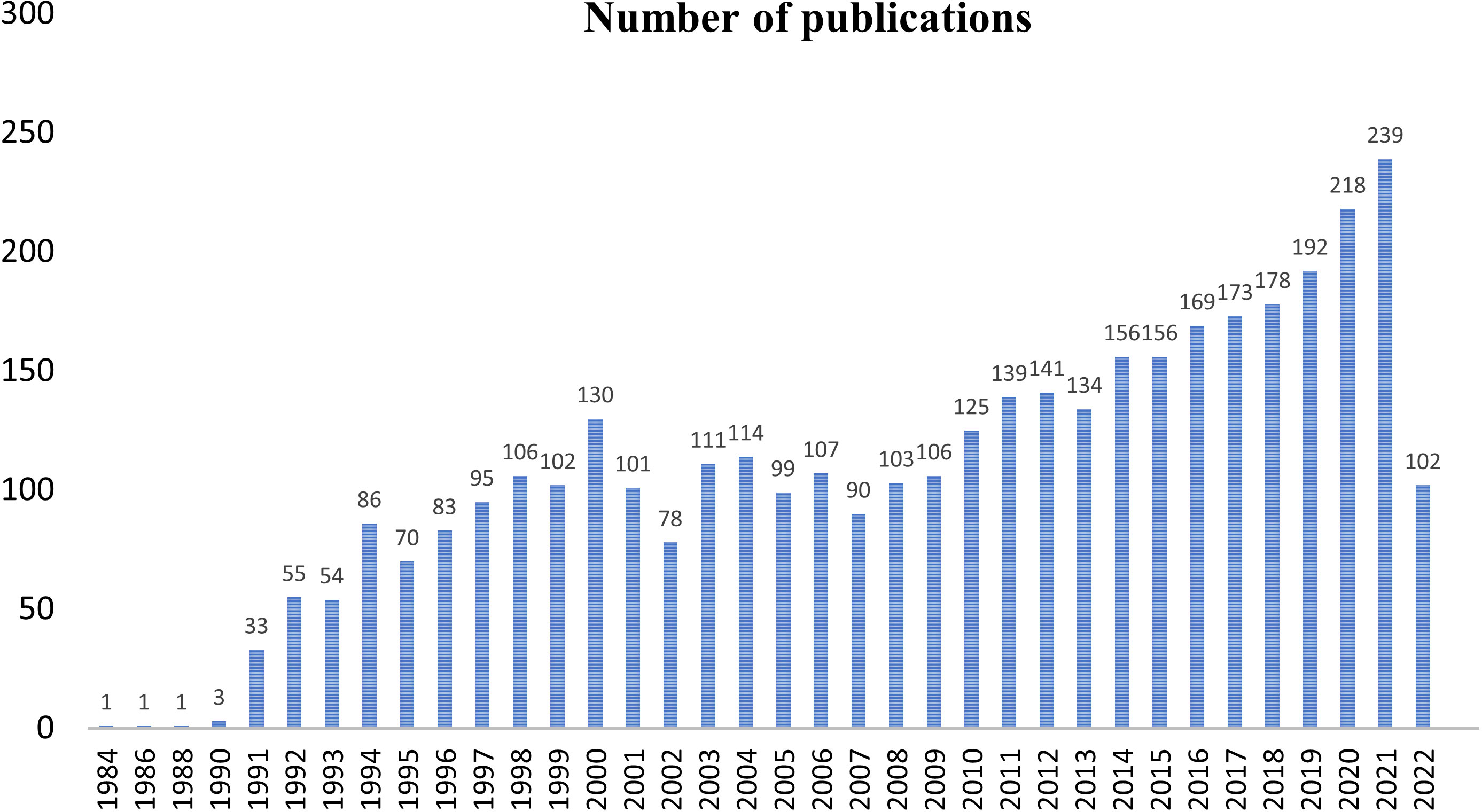
The first query aimed to overview Ca2+ and inflammation research in immune cells in the current literature. In total, 3865 articles were published between 1984 and 2022. An average of 90 publications per year between 1990 and 2010 were reported. Over the next ten years, publications increased strongly, up to 2-fold from 2010 to 2021. Analysis was done until July 2022, which explains the lowest number of publications for this year (Figure 2). Most of them were published in the United States of America and China and, to a lesser extent, in Europe, Canada, and South America (Figure 3). However, the number of collaborations between all these countries was important. Collaborations for these articles were made mainly between the USA, European countries, and China, indicating a worldwide interest in the Ca2+ and inflammation topic in immune cells (Figure 4).
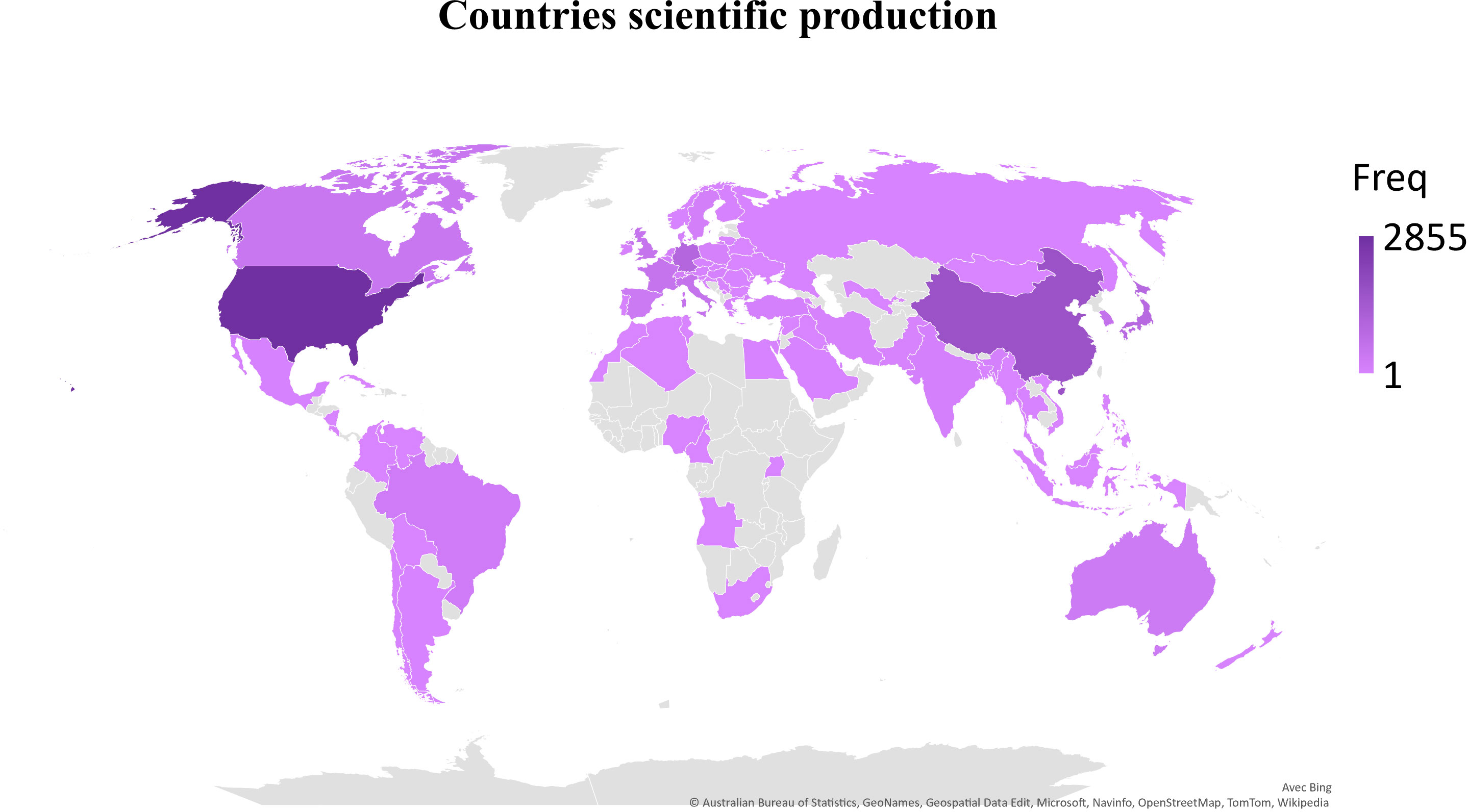
Figure 3 Scientific production by country, displayed by the total number of publications over the query time (Freq).
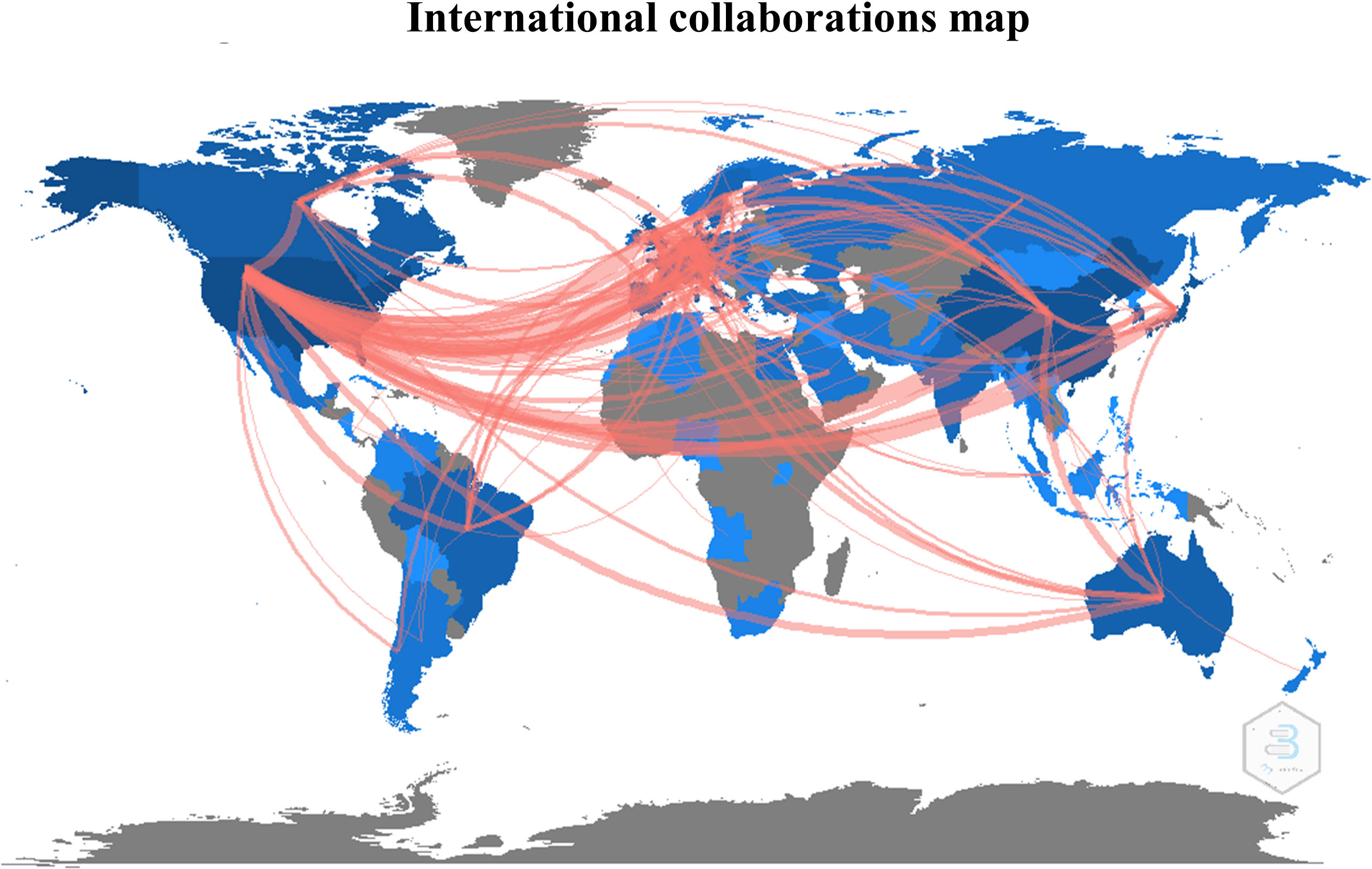
Figure 4 International collaborations map, represented by the number of collaborative studies between countries.
3.2 Co-citation analysis
The co-citation analysis provides an overview of the most substantial contributions to the field by measuring the frequency of two articles being cited together in a scientific literature corpus (19). The analysis was done on the 133 most co-cited documents grouped into 4 clusters. This threshold highlights the most influential articles. The cluster denomination was based on articles from the previously described selection method. The co-citation network is displayed in Figure 5. The cluster position provides information about the relationship between topics. We observed the closeness of the red and yellow clusters and their remote connection to the blue and green clusters. The content of each cluster will be further developed to provide interpretation resources on the relations between these groups.
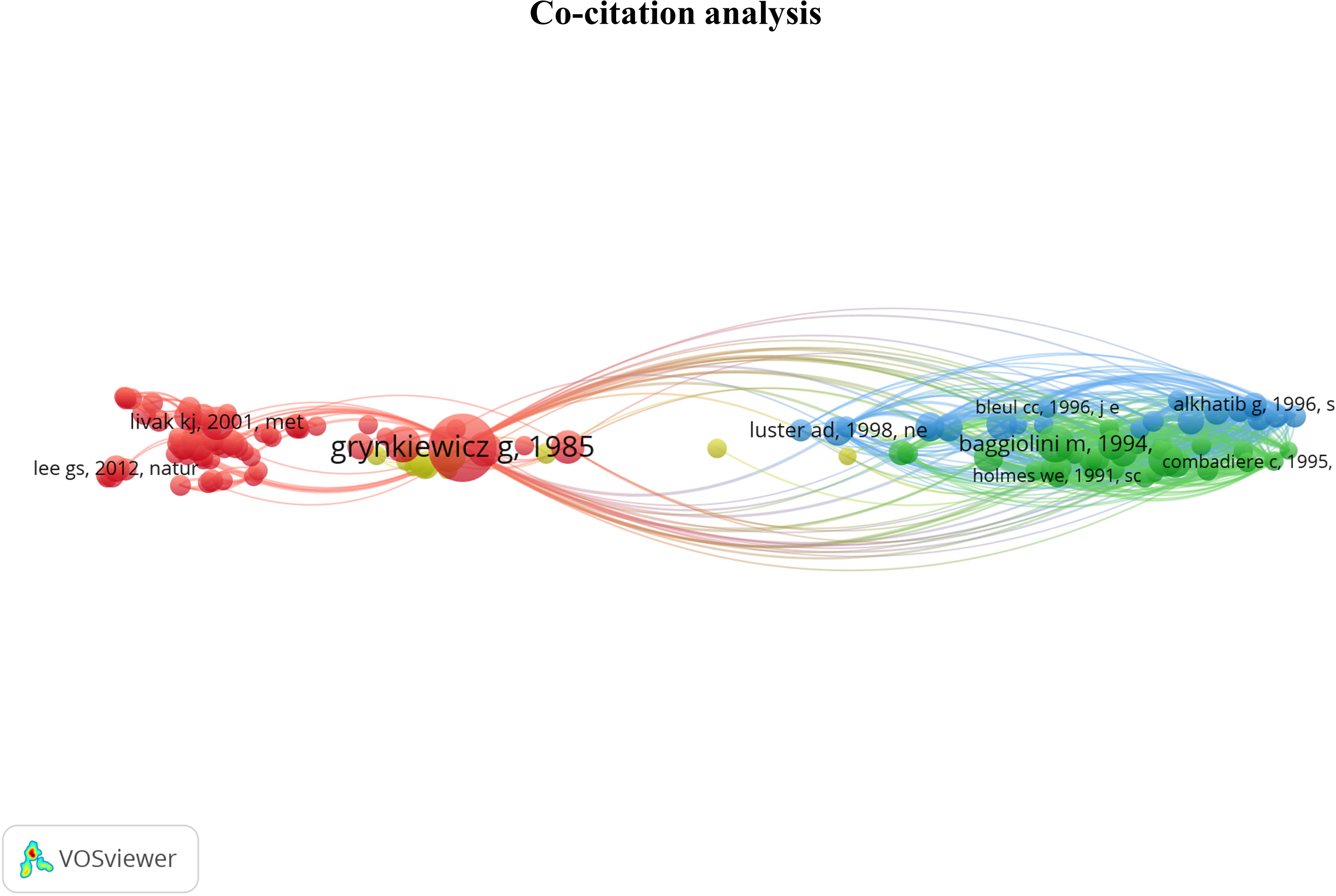
Figure 5 Co-citation network providing an overview of the most contributing publications in the Ca2+ and inflammation context. Red cluster [1976 - 2006]: Development of Ca2+ probes, Ca2+ signaling, and channels identified in the plasma membrane. Yellow cluster [1987 - 1997]: intracellular pathways & calcium-binding proteins; Green cluster [1991 - 1995]: chemokine receptors; Blue cluster [1995 - 2006]: RANTES chemokines in HIV.
In the red cluster, we observed two groups; one has a central position and is related to all the other clusters, i.e., yellow, green, and blue clusters. In the red cluster, 17 out of 68 publications were in the 3rd quartile, ranging from 1976 to 2012. The red cluster relates to Ca2+ signaling and channels in the plasma membrane. Most publications are focused on the Stromal Interaction Molecule (STIM), a Ca2+ sensor essential for the SOCE through its binding to ORAI1 (Calcium Release-Activated Calcium Modulator 1). ORAI proteins are STIM-binding partners that form the channel pore in the plasma membrane (22, 23). These channels represent the main pathway of Ca2+ influx in T- and B-cells and promote the immune response by partly activating the transcription factor nuclear factor of activated T-cells (NFAT) (14, 24, 25). The study by Grynkiewicz et al., published in 1985 (26), appears in a central position. This article deals with a new generation of highly fluorescent indicators to study the physiological role of cytosolic free Ca2+ concentration, with greatly improved fluorescence properties. Most articles in the second part of the red cluster use the probes described in Grynkiewicz’s article, notably Fura2-AM (27–29). Therefore, this cluster highlights the fundamental knowledge of Ca2+ signaling in PBMCs.
Near the red cluster, the yellow cluster includes 3 out of 14 publications in the 3rd quartile ranging from 1987 to 1991. They refer to calcium-binding proteins (CaBPs), such as the S100 protein. S100 was reported to be associated with specific stages of monocyte differentiation (30, 31). S100 function is still unclear, but some evidence suggests that macrophages infiltrated during inflammation express the myeloid-related protein 8 (MRP8) and the myeloid-related protein 14 (MRP-14) members of the S100 protein family in rheumatoid arthritis pathology (32). These two clusters related to the Ca2+ topic support the critical role of Ca2+ signaling in immune cell function.
The last two clusters, green and blue, are located on the opposite side of the maps and share close positions and content. No temporal evolution was observed in these two clusters, since all the articles were published in the 90s (33–36). The green cluster contains 7 out of 30 articles in the 3rd quartile, ranging from 1991 to 1995, which refer to chemokine receptors involved in inflammation. In its vicinity, the blue cluster with 5 out of 21 articles in the 3rd quartile, from 1995 to 1996, focuses on chemokines, especially the regulated-on activation, normal T cell expressed and secreted (RANTES) or chemokine ligand 5 (CCL5) axis in HIV infections. Indeed, RANTES research was achieved at the time of the outbreak emergence of HIV at the beginning of the 1980s, instigating extensive research on this chemokine.
To conclude, the co-citation analysis supports the crucial role of Ca2+ and chemokine signaling in the immune cell function, which depends on signal integration, stress, chemical environment, and inflammation, key features of several pathologies. More precisely, all these parameters lead to modulation and cell activation by intracellular pathways, activating CaBPs and partially leading to their translocation to the nucleus. The latter activates Ca2+-dependent transcription factors that control PBMC functions such as proliferation, differentiation, and cytokine production.
3.3 Bibliographic coupling analysis
Bibliographic coupling is based on the idea that two publications sharing common references have similar content. This analysis enables the formation of thematic clusters in the literature corpus obtained with our query. Consequently, recent niche publications may appear in our analysis. This section aimed to determine what drove the calcium and inflammation research field on PBMCs over the last three decades. The analysis was done on 998 articles, and 8 clusters were identified (Figure 6). The large number of articles and the link between them make the interpretation complex; a preliminary exploration of the clusters showed a large representation of pathologies involving an immune response, and the in-depth analysis of the clusters provided further knowledge on the field structure.
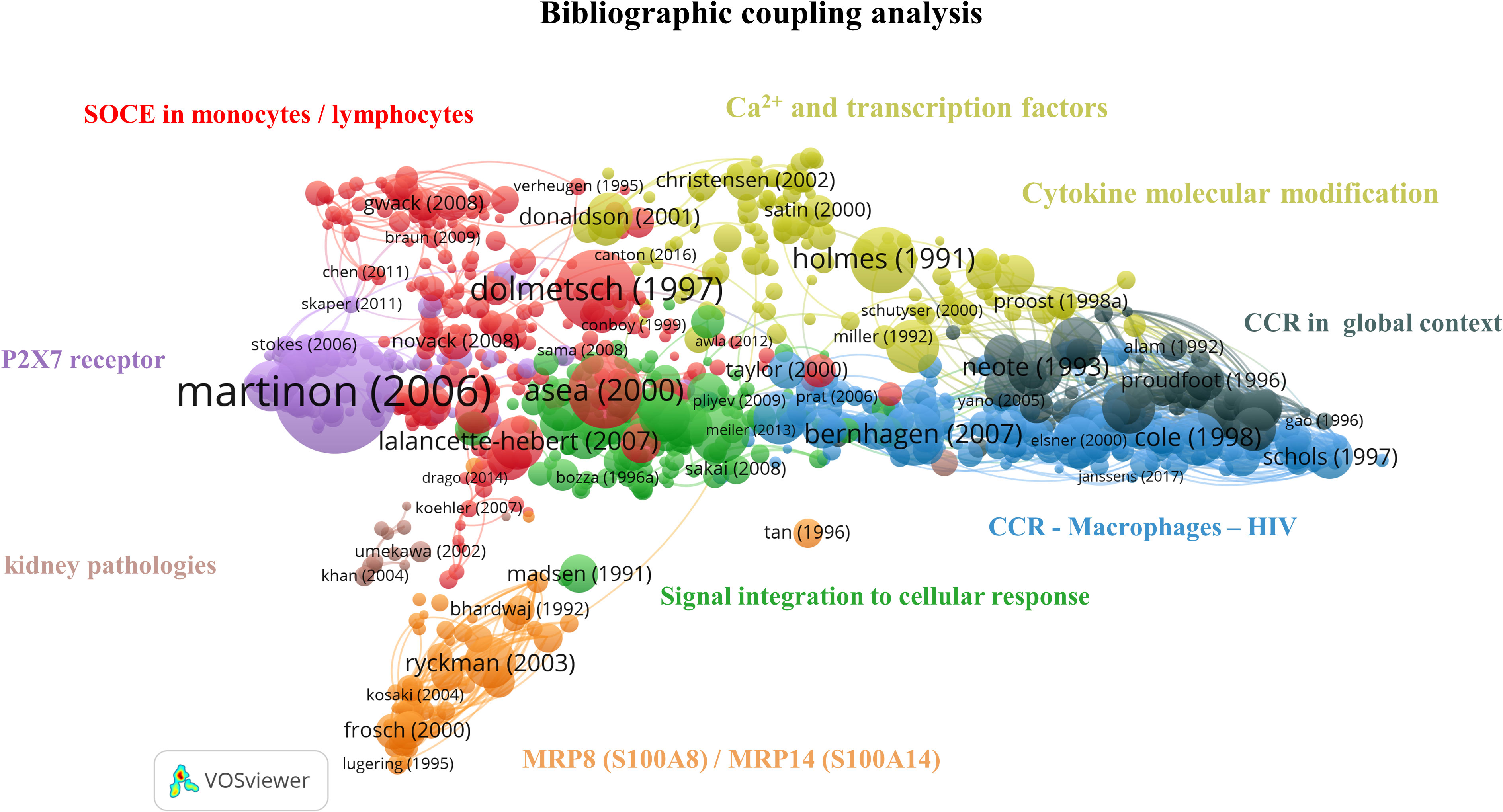
Figure 6 Bibliographic coupling network: Ca2+ and inflammation in PBMCs [1990-2023]. The red cluster represents the CRAC channel in lymphocytes and monocytes. The purple cluster focuses on the P2X7 purinergic receptor in macrophages. The orange cluster refers to the specific calcium-binding protein MRP8 (S100A8)/MRP14 (S100A14). The green cluster covers the cascade from signal integration to cellular response. The yellow cluster relates to Ca2+ signaling, transcription factors and cytokine molecular modifications. The black cluster targets chemokine receptors, the blue cluster accounts for chemokine receptors in HIV infections, and the brown cluster focuses on kidney pathologies.
The red cluster contains 57 out of 232 articles in the 3rd quartile and refers to the SOCE. This cluster can be further divided into subgroups related to lymphocytes and macrophages, respectively.
We identified articles about the calcium release-activated channel (CRAC) components in the lymphocyte cluster. In 2005 – 2006, both STIM1 Ca2+ sensor and ORAI1 channel-forming protein were determined by Feske et al. The CRAC composition depends on cell type, localization, and activation state, but ORAI1 is the dominant channel member in several immune cells, mainly in neutrophils and mast cells (37–39). In physiological conditions, the CRAC channel is activated by inositol 1,4,5 phosphate (IP3) binding to IP3 receptors (IP3R) in the endoplasmic reticulum (ER) membrane following TCR or BCR activation. IP3 leads to IP3R opening, resulting in Ca2+ release from the ER into the cytosol. The resulting ER Ca2+ decrease induces a conformational change of STIM1, which binds to ORAI1 channels to open its pore in the plasma membrane. The importance of Ca2+ influx through plasma membrane channels in T cells was characterized by studying the Ca2+ conductance triggered by the TCR. An absence of this current was demonstrated in human T cells from patients with immunodeficiency diseases (16). Missense mutations in ORAI1 affect the channel function and the subsequent T-cell function leading to severe immunodeficient phenotypes (40, 41).
In non-excitable cells such as lymphocytes, potassium (K+) channels were identified in 1983 by Matteson and Deutschand in 1984 by DeCoursey et al. The latter publication shows their implication in functional processes such as mitogenesis (42). In T-lymphocytes, the engagement of the TCR/CD3 complex upon antigen binding leads to the increase of intracellular Ca2+. This Ca2+ influx is maintained by K+ channels through K+ release outside the cells, preserving the electrochemical potential gradient. Panyi et al. reported that the interleukin-2 (IL-2) production and cell proliferation in T cells are partly mediated by K+ channels (43). The inhibition of the K+ channel, named Kv1.3, inhibits T-cell activation, calcium signaling, cytokine production, and cell proliferation (44).
STIM1 and ORAI proteins were also identified in phagocytic immune cells, notably in macrophages, as demonstrated in papers in the related sub-clusters (38). There are several phenotypes of macrophages: the pro-inflammatory one, also known as classical monocytes (M1), with an important phagocytosis function; and the anti-inflammatory one as non-classical monocytes (M2). Macrophage plasticity is essential for innate immunity since macrophages can switch their phenotype according to their chemical environment. Chauban et al. showed that ORAI1 significantly contributes to Ca2+ entry in vitro using non-differentiated macrophages (M0). In contrast, in vitro M1 polarization induced by IFNɣ is associated with the recruitment of the transient receptor potential cation channel 1 (TRPC1) to enhance Ca2+ entry, leading to high expression of inflammatory genes (45).
Additionally, this subcluster references the monovalent cation channel transient receptor potential melastatin (TRPM), involved in the physiological response in some immune cells, i.e., monocytes and macrophages, through intracellular Ca2+ level regulation. High intracellular Ca2+ concentration leads to TRPM4 opening, regulating Na+ entry and Ca2+ efflux. Indeed, TRPM4 works in concert with the CRAC channel to achieve this regulation. Serafini et al. studied TRPM4 deletion in a sepsis mouse model. They observed an altered function in the absence of TRPM4 through a decrease in phagocytosis and an increased pro-inflammatory cytokine production, leading to an alteration of macrophage function affecting the mouse survival (46).
In summary, the red cluster highlights the central role of Ca2+ signaling in the immune function of macrophages and lymphocytes. It therefore represents the largest cluster in our query, gathering around 23% of the articles in the bibliographic coupling map.
Close to the red cluster, the main topic of the purple cluster is centered on purinergic receptors, such as P2X7, and includes 22/89 articles in the 3rd quartile, from 2003 to 2016. It is well known that Ca2+ signaling enhances mitochondrial adenosine triphosphate (ATP) production in activated T cells (14). ATP is then exported outside T cells through the Pannexin 1 channel and activates P2X7, which causes further Ca2+ entry (14). P2X7 receptors are expressed on mast cells, lymphocytes, erythrocytes, fibroblasts, and peripheral macrophages. In monocytes/macrophages, P2X7 receptor activation leads to interleukin production, notably interleukin 1-bêta (IL-1β) as a pro-inflammatory factor (47).
The orange cluster contains 14/57 publications in the 3rd quartile and is localized in the network periphery. It refers to the specific CaBP S100 (48), an inflammatory marker, thus explaining its remote connection to the other inflammation-themed clusters (49).
In the green cluster, 27 articles cover the role of intracellular pathways in immune cells, including Ca2+ fluxes, receptors, and transcription factors such as nuclear factor kappa B (NF-κB) (50–52). The remaining 7 publications focus on cytokines, key soluble elements of signal integration in cellular stress (53–59). Therefore, this cluster gives an overview of immune cell signal integration from the cytokine binding to its receptor up to the gene expression, explaining its central position on this bibliographic coupling map.
The yellow cluster is widely extended and includes 32/129 articles in the 3rd quartile. A part of it is located close to the red cluster and covers articles related to Ca2+ signaling and transcription factors (60, 61). In contrast, the other part contains those related to cytokine molecular modification (62–64). This latter part is close to the black cluster that contains 14/58 publications in the 3rd quartile and focuses on chemokine receptors (CCR) and their ligand. Chemokine receptors are G-protein-coupled-receptors serpentine receptors. Chemokines, a particular type of small cytokines, are known to contribute to the trafficking of leukocytes to the inflammation site through a signaling cascade. Depending on the environment, chemokines activate neutrophils to attract and activate monocytes, basophils, eosinophils, or lymphocytes (65). Several subclasses of chemokine receptors exist and are expressed constitutively or induced by inflammation (66). Moreover, some receptors can bind specific ligands or several chemokines. Thus, chemoattractants possess a crucial regulatory role in immunity and are involved in viral infection (67).
The blue cluster, with 53/210 articles in the 3rd quartile, includes articles from 1997 to 2004, correlating with the HIV epidemic starting in 1981, and completes the preceding cluster by specifying the role of chemokine receptors in HIV infection. The HIV virion targets the cellular plasma membrane, and the fusion reaction occurs thanks to the viral envelope glycoprotein, which binds the CD4 surface marker (68). The virus uses the CXCR4 and CCR5 receptors for T cells and macrophages, respectively. The CXCR4 ligand is a natural ligand identified as stromal cell-derived factor 1 (SDF-1), also known as chemokine-12 (CXCL-12), and has the properties of a selective inhibitor of T cell tropism (69). Moreover, in 1995, Lusso et al. indicated that the CCL5 or RANTES cytokine, the macrophage inflammatory proteins 1α (MIP-1α/CCL3) and 1β (MIP-1β/CCL4) were all HIV-suppressive factors released by CD8+ T cells (70, 71).
The brown cluster focuses on kidney pathologies, especially on calcium oxalate crystal formation and on the role of monocytes-macrophages and the chemokine ligand 2 (CCL2), also known as monocyte chemoattractant protein 1 (MCP-1), in this pathology. This cluster contains 2/15 publications in the 3rd quartile with two different topics. The first one investigates macrophage capacity to suppress renal crystal formation (72). At the same time, the second one refers to the CCL2 role in tubulointerstitial inflammation during kidney failure by inducing cytokine and adhesion molecule production (73). Of note, this cluster does not appear as the most relevant for the bibliometric coupling map analysis due to its low article number and its remote location and topic from other clusters mainly focused on Ca2+ signaling.
Altogether, this bibliographic coupling showed that Ca2+ signaling is widely studied in physiological conditions and several pathologies involving an immune response. Since intracellular Ca2+ signaling partly controls cytokine production, it was expected to see such a coupling between the Ca2+ signaling clusters and the inflammatory ones. Therefore, the resulting map aligns with our current field knowledge.
3.4 Ca2+ analysis by flow cytometry
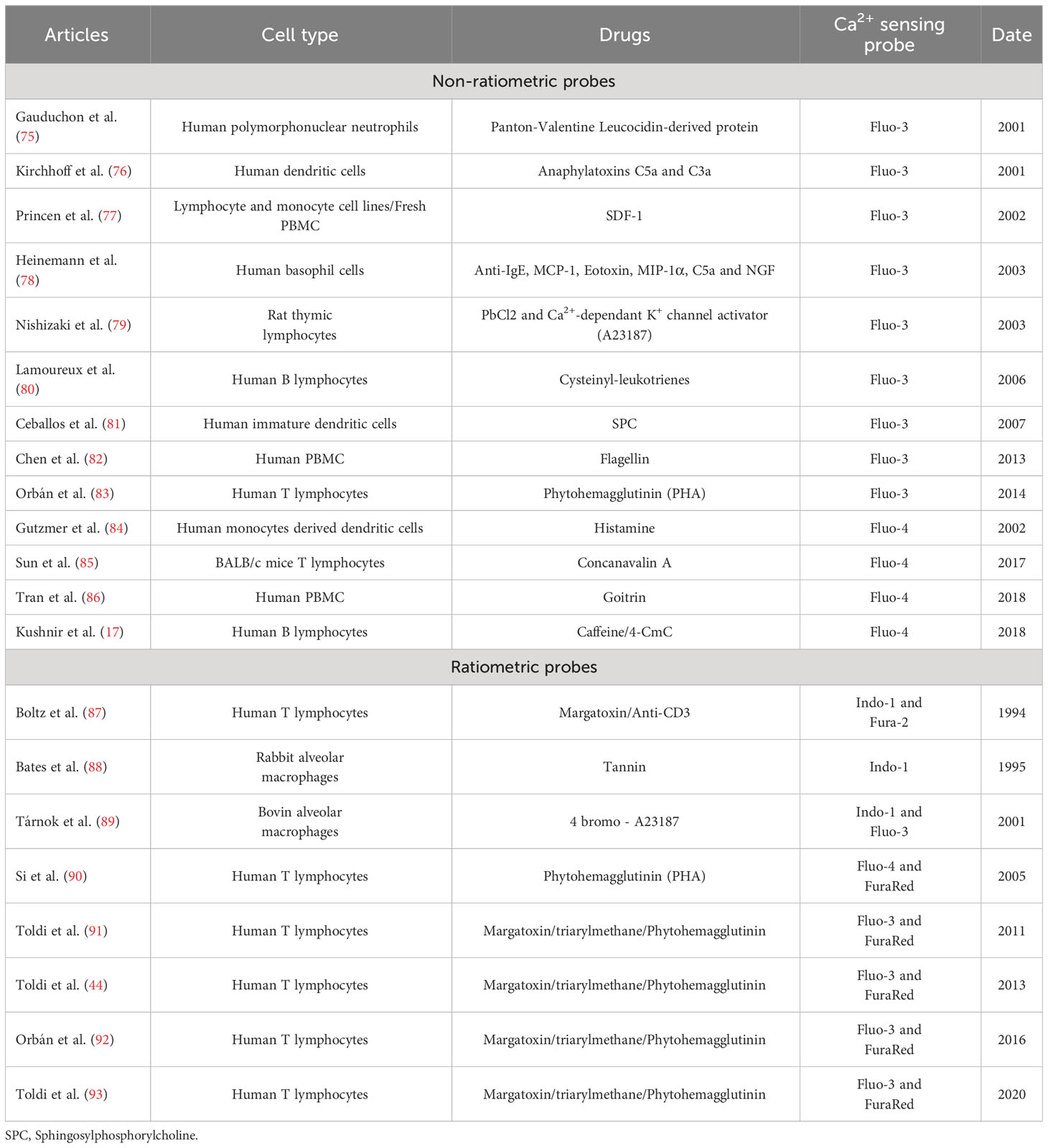
Immunophenotyping using flow cytometry has become the gold standard method used in clinical (74) and fundamental research laboratories to characterize immune cells derived from patient blood samples. As the bibliographic coupling reveals, Ca2+ signaling is crucial in controlling immune cell functions. We next wanted to determine the evolution of flow cytometry-based studies to evaluate the Ca2+ kinetics in PBMCs over the last three decades. To perform this analysis, another query was defined to focus on flow cytometry, as specified in the “Materials and Methods” section. Ranging from 1993 to 2022, we obtained 184 articles. These articles were split into two flow cytometry usage categories: (1) for the phenotyping of immune cell profiles and (2) to analyze Ca2+ kinetics in PBMCs. We reported and focused our review on the 21 publications falling in the latter category (Table 1).
Regarding the cell compartment analyzed, all the publications obtained by this query referred to the cytosolic Ca2+ pool. Among them, 13 articles focused on cytosolic Ca2+ levels using the non-ratiometric probes Fluo3 or Fluo4, thus not allowing any resting Ca2+ level measurement. The other 8 articles performed ratiometric measurements, mainly through the association of Fluo3 and FuraRed probes: the ratio between the green fluorescence of Fluo3 over the red fluorescence of FuraRed, respectively increasing and decreasing when bound to Ca2+, was used as a ratiometric measurement of cytosolic Ca2+ level (93, 94). Indeed, ratiometric fluorescent Ca2+ indicators minimize the effects of photobleaching, leakage, and uneven loading delivery, allowing more robust and reproducible results. Over the last decades, the combination of Fluo3 and FuraRed has been performed in flow cytometry, displaying a greater response magnitude than the ratiometric probe Indo1 requiring ultraviolet excitation, which is often unavailable on flow cytometers (94). It has been adapted to other cell types, such as platelets (78). Importantly, as FuraRed is a stand-alone ratiometric probe, its single-use showed similar efficiency in ratiometric measurements of cytosolic Ca2+ level in PBMCs (95). The single-use of FuraRed, compared to its combination with Fluo3, enables additional cell subtype labeling through additional channels with a faster, cheaper, and more accurate preparation (loading of only one dye).
Regarding the stimulation employed for the Ca2+ signaling pathways, the authors focused mainly on extracellular stimulation through the plasma membrane modulation of TCR/BCR or K+ channels. Notably, Toldi et al. used flow cytometry to understand how potassium channel inhibition impacts calcium influx in human T lymphocytes of patients with autoimmune disorders, using triarylmethane as a specific inhibitor of IKCa1 channel (44). Flow cytometry also enabled multiparametric analyses to study Ca2+ fluxes in T cell subpopulations even in low abundance, i.e., CD4+/CD8+/CD4+/CXCR3+ or CD4/CCR4 + (91, 93).
Overall, this query highlights that the study of Ca2+ signaling could rely more on flow cytometry. However, the trend is increasing over the last decade (8 out of 21 publications published after 2013). As for the techniques employed, both non-ratiometric and ratiometric probes are used, mainly to assess cytosolic Ca2+ levels, therefore putting aside several actors of Ca2+ signaling in other cell compartments. From this particular query, only one paper performed intracellular stimulations on a reticular player: Kushnir et al. (17) focused on the major ER Ca2+ release channel, ryanodine receptor (RyR), using caffeine as an agonist. They specifically looked at the cytosolic Ca2+ level with Fluo4 in human B lymphocytes targeted with an anti-CD19 antibody in PBMCs. The Ca2+ response amplitudes obtained by a 50 mM caffeine stimulation were recorded in normal or congestive heart failure lymphocytes and revealed an ER Ca2+ leakage as a signature of the pathology.
4 Discussion & perspectives
Our literature review query on inflammation and Ca2+ signaling revealed 3865 articles published between 1984 and 2022, which supported the design of a bibliometric analysis. We reported that conventional flow cytometry is extensively used in clinical routine as a tool for diagnosis and monitoring of inflammatory diseases. A significant advantage of this method lies in the compensation process to correct fluorescent spillovers, increasing the number of parameters studied and enabling target analysis of specific cell populations. Clinical biomarkers of inflammatory pathologies were identified with this quantitative multi-parametric analysis. More recently, spectral flow cytometry collecting the full light spectrum has enabled the distinction of unique fluorophores with overlapping emission spectra (96, 97). Alongside with technological improvements, ratiometric chemical probes have steadily evolved since the 1980s (26, 95).
Regarding Ca2+ signaling, we highlighted that Ca2+ players involved in human PBMC models were studied and described in physiological settings. Indeed, Ca2+ ions are ubiquitous intracellular second messengers with a crucial role in the immune cell function: from the cell-specific membrane receptor activation to the SOCE, all contributing to the transcription of immune response genes (Figure 7). Therefore, flow cytometry represents a powerful tool compatible with immunophenotyping, allowing the analyses of Ca2+ fluxes in immune cell subtypes, even when underrepresented. So far, we have reported only 21 articles using flow cytometry to study Ca2+ signaling focused on cytosolic Ca2+ level variations bypassing the contribution of organelles, notably the ER as the main Ca2+ store and mitochondria whose function depends, in part, on Ca2+ signaling. Interestingly, a Ca2+ coupling exists between ER and mitochondria to control mitochondrial functions. In this regard, Assis et al. suggested that the width of ER-mitochondria coupling was associated with the macrophage activation status (98). However, whether this structural modification translates into functional consequences on the Ca2+ transfer from the ER to mitochondria requires further investigation. Additionally, a recent study demonstrated a reduced mitochondrial Ca2+ uptake via the mitochondrial Ca2+ uniporter during aging in macrophages as a potential contributor to inflammation in humans (99). The Ryanodine receptor is expressed in immune cells and regulates intracellular Ca2+ homeostasis. Osipchuk et al. reported that pharmacological inhibition of RyR leads to intracellular Ca2+ alteration and alters immune cell functions in vitro and in vivo (100). In PBMCs from heart failure patients, Kushnir et al. (17) reported an ER Ca2+ leakage in B cells as a biomarker of this pathology. Regarding the SERCA pump, its role is yet to be described in PBMCs. Therefore, the contribution of the different organelles, notably ER, mitochondria, and their coupling, in regulating the immune cell function remains to be deciphered. Albeit combining two Ca2+ chemical probes to study two cellular compartments simultaneously remains unexplored. However, with the increasing development of new ratiometric sensors targeting several cell compartments, multiparametric analyses of Ca2+ fluxes by flow cytometry may become a promising alternative for human immune cell research in the coming decades.
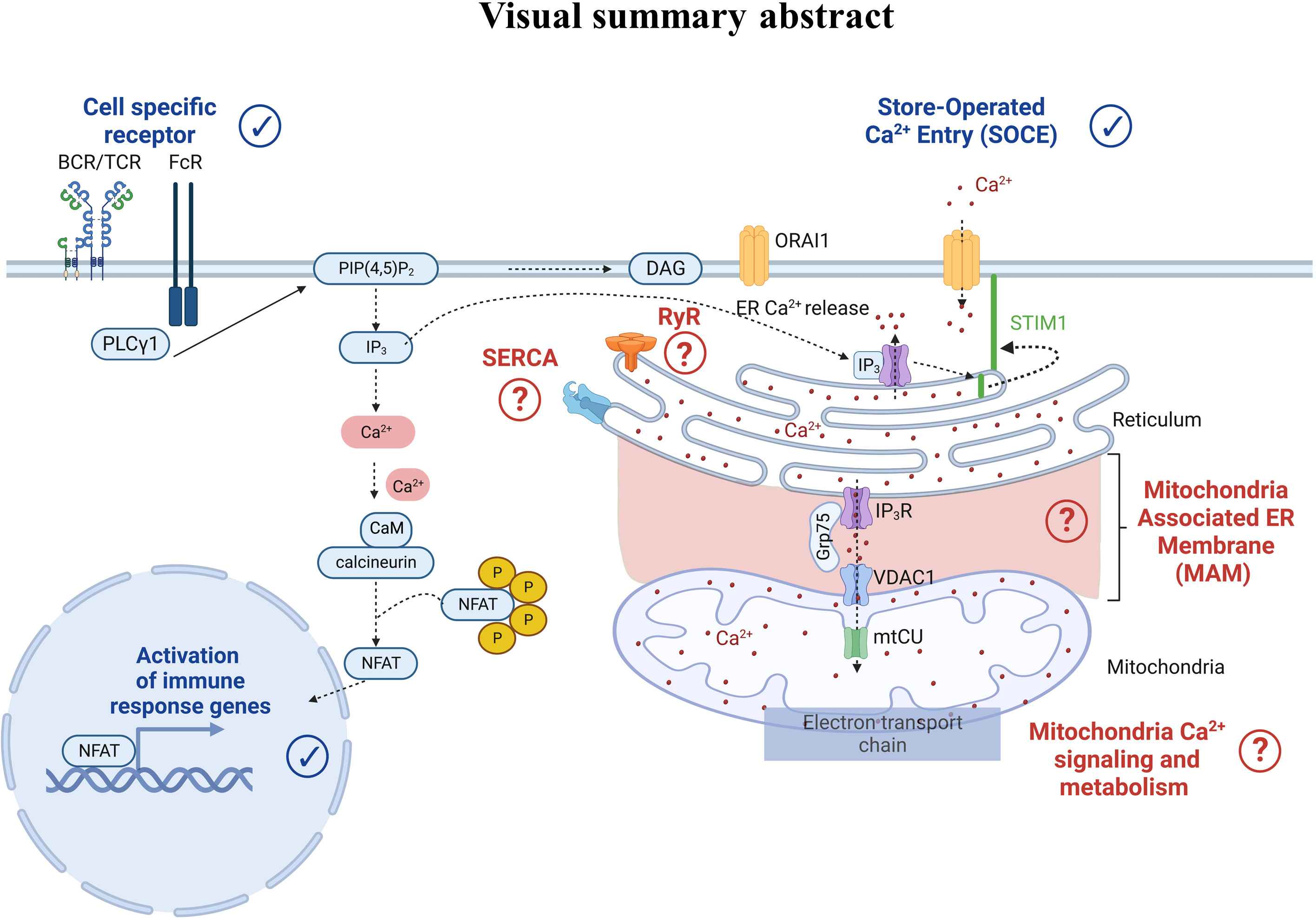
Figure 7 Cell-specific receptor activation through antigens induces protein kinase phosphorylation and phospholipase C gamma 1 activation. The latter leads to the production of the second messenger inositol-1,4,5-triphosphate (IP3), which binds to the IP3 receptor (IP3R) in the endoplasmic reticulum (ER) membrane. IP3R activation induces Ca2+ efflux from the ER to the cytosol. As a result, an increase of the cytosolic Ca2+ concentration and a decrease of the ER Ca2+ concentration occur. Sensors in the ER called Stromal Interaction Molecule 1 (STIM1) detect the ER Ca2+ decrease, leading to STIM1 oligomerization and translocation to the plasma membrane where it binds to the ORAI1 protein. STIM1-ORA1 interactions contribute to Ca2+ influx which elevates the intracellular Ca2+ concentration leading to the restoration of the ER Ca2+ stocks via repumping through the Sarco-Endoplasmic Reticulum Calcium ATPase (SERCA). In parallel, the increase in cytosolic Ca2+ concentration also triggers the calcineurin- NFAT pathways. More recently, Ca2+ players such as ryanodine receptors (RyR), SarcoEndoplasmic Reticulum Calcium ATPase (SERCA), the IP3R-Grp75-VDAC complex, and the mitochondrial Ca2+ uniporter (mtCU) have emerged as potential contributors to the Ca2+ signaling pathway, requiring further dedicated research.
Author contributions
CB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. LC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – review & editing. FM: Conceptualization, Formal Analysis, Investigation, Writing – review & editing. TB: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing. SD: Conceptualization, Supervision, Validation, Writing – review & editing. MP: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing. CCDS: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was funded by grants from the Fondation de l’Avenir (n° AP-RM-20-008), from the French National League of Basketball, from the Agence Nationale de la Recherche (ANR- 20-CE14-0013-01) and Fédération Française de Cardiologie (FFC-BOCHATON-Dotation 2020). The Ph.D. salary of LC (Cifre, Olea Medical) is co-funded by the French Ministry of Higher Education and Research (ANRT).
Acknowledgments
We would like to thank the members of IRIS team for their fruitful discussions.
Conflict of interest
LC was employed by Olea Medical.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1272809/full#supplementary-material
References
1. Finak G, Langweiler M, Jaimes M, Malek M, Taghiyar J, Korin Y, et al. Standardizing flow cytometry immunophenotyping analysis from the human immunoPhenotyping consortium. Sci Rep (2016) 6:20686. doi: 10.1038/srep20686
2. Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. J Immunol Methods (2000) 243:77–97. doi: 10.1016/S0022-1759(00)00229-5
3. McKinnon KM. Flow cytometry: an overview. Curr Protoc Immunol (2018) 120:5.1.5. doi: 10.1002/cpim.40
4. Barnett D, Walker B, Landay A, Denny TN. CD4 immunophenotyping in HIV infection. Nat Rev Microbiol (2008) 6:S7–15. doi: 10.1038/nrmicro1998
5. Bridts CH, Sabato V, Mertens C, Hagendorens MM, De Clerck LS, Ebo DG. Flow cytometric allergy diagnosis: basophil activation techniques. In: Gibbs BF, Falcone FH, editors. Basophils and Mast Cells: Methods and Protocols. New York, NY: Springer (2014). p. 147–59.
6. Li W. Flow cytometry in the diagnosis of leukemias, in: Leukemia (2022). Brisbane (AU: Exon Publications. Available at: http://www.ncbi.nlm.nih.gov/books/NBK586209/ (Accessed 7 May 2023).
7. Coppola L, Smaldone G, D’aiuto M, D’aiuto G, Mossetti G, Rinaldo M, et al. Identification of immune cell components in breast tissues by a multiparametric flow cytometry approach. Cancers (2022) 14:3869. doi: 10.3390/cancers14163869
8. Lopresti A, Malergue F, Bertucci F, Liberatoscioli ML, Garnier S, DaCosta Q, et al. Sensitive and easy screening for circulating tumor cells by flow cytometry. JCI Insight (2019) 5:e128180, 128180. doi: 10.1172/jci.insight.128180
9. Bourgoin P, Soliveres T, Ahriz D, Arnoux I, Meisel C, Unterwalder N, et al. Clinical research assessment by flow cytometry of biomarkers for infectious stratification in an Emergency Department. Biomark Med (2019) 13:1373–86. doi: 10.2217/bmm-2019-0214
10. Amin J, Boche D, Clough Z, Teeling J, Williams A, Gao Y, et al. Peripheral immunophenotype in dementia with Lewy bodies and Alzheimer’s disease: an observational clinical study. J Neurol Neurosurg Psychiatry (2020) 91:1219–26. doi: 10.1136/jnnp-2020-323603
11. Pellicanò M, Larbi A, Goldeck D, Colonna-Romano G, Buffa S, Bulati M, et al. Immune profiling of Alzheimer patients. J Neuroimmunol (2012) 242:52–9. doi: 10.1016/j.jneuroim.2011.11.005
12. Obasanmi G, Lois N, Armstrong D, Hombrebueno JMR, Lynch A, Chen M, et al. Peripheral blood mononuclear cells from patients with type 1 diabetes and diabetic retinopathy produce higher levels of IL-17A, IL-10 and IL-6 and lower levels of IFN-γ—A pilot study. Cells (2023) 12:467. doi: 10.3390/cells12030467
13. Feske S. Calcium signaling in lymphocyte activation and disease. Nat Rev Immunol (2007) 7:690–702. doi: 10.1038/nri2152
14. Trebak M, Kinet J-P. Calcium signalling in T cells. Nat Rev Immunol (2019) 19:154–69. doi: 10.1038/s41577-018-0110-7
15. Nunes P, Demaurex N. The role of calcium signaling in phagocytosis. J Leukoc Biol (2010) 88:57–68. doi: 10.1189/jlb.0110028
16. Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem (1994) 269:32327–35. doi: 10.1016/S0021-9258(18)31639-9
17. Kushnir A, Santulli G, Reiken SR, Coromilas E, Godfrey SJ, Brunjes DL, et al. Ryanodine receptor calcium leak in circulating B-lymphocytes as a biomarker in heart failure. Circulation (2018) 138:1144–54. doi: 10.1161/CIRCULATIONAHA.117.032703
18. Bajnok A, Serény-Litvai T, Temesfői V, Nörenberg J, Herczeg R, Kaposi A, et al. An optimized flow cytometric method to demonstrate the differentiation stage-dependent Ca2+ Flux responses of peripheral human B cells. Int J Mol Sci (2023) 24:9107. doi: 10.3390/ijms24109107
19. Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: An overview and guidelines. J Bus Res (2021) 133:285–96. doi: 10.1016/j.jbusres.2021.04.070
20. Chalet L, Boutelier T, Christen T, Raguenes D, Debatisse J, Eker OF, et al. Clinical imaging of the penumbra in ischemic stroke: from the concept to the era of mechanical thrombectomy. Front Cardiovasc Med (2022) 9:861913. doi: 10.3389/fcvm.2022.861913
22. Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol CB (2005) 15:1235–41. doi: 10.1016/j.cub.2005.05.055
23. Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S-H, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature (2006) 441:179–85. doi: 10.1038/nature04702
24. Vig M, Kinet J-P. Calcium signaling in immune cells. Nat Immunol (2009) 10:21–7. doi: 10.1038/ni.f.220
25. Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol (2008) 20:250–8. doi: 10.1016/j.coi.2008.04.004
26. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem (1985) 260:3440–50. doi: 10.1016/S0021-9258(19)83641-4
27. Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci (1993) 90:6295–9. doi: 10.1073/pnas.90.13.6295
28. Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature (1997) 386:855–8. doi: 10.1038/386855a0
29. Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev (2005) 85:757–810. doi: 10.1152/physrev.00057.2003
30. Teigelkamp S, Bhardwaj RS, Roth J, Meinardus-Hager G, Karas M, Sorg C. Calcium-dependent complex assembly of the myeloic differentiation proteins MRP-8 and MRP-14. J Biol Chem (1991) 266:13462–7. doi: 10.1016/S0021-9258(18)98862-9
31. Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem (1991) 266:7706–13. doi: 10.1016/S0021-9258(20)89506-4
32. Odink K, Cerletti N, Brüggen J, Clerc RG, Tarcsay L, Zwadlo G, et al. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature (1987) 330:80–2. doi: 10.1038/330080a0
33. Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell (1996) 85:1135–48. doi: 10.1016/s0092-8674(00)81313-6
34. Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, et al. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell (1996) 85:1149–58. doi: 10.1016/S0092-8674(00)81314-8
35. Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature (1996) 381:661–6. doi: 10.1038/381661a0
36. Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature (1996) 381:667–73. doi: 10.1038/381667a0
37. Bogeski I, Kummerow C, Al-Ansary D, Schwarz EC, Koehler R, Kozai D, et al. Differential redox regulation of ORAI ion channels: A mechanism to tune cellular calcium signaling. Sci Signal (2010) 3:ra24–4. doi: 10.1126/scisignal.2000672
38. Demaurex N, Nunes P. The role of STIM and ORAI proteins in phagocytic immune cells. Am J Physiol-Cell Physiol (2016) 310:C496–508. doi: 10.1152/ajpcell.00360.2015
39. Ambudkar IS, de Souza LB, Ong HL. TRPC1, Orai1, and STIM1 in SOCE: Friends in tight spaces. Cell Calcium (2017) 63:33–9. doi: 10.1016/j.ceca.2016.12.009
40. Wenning AS, Neblung K, Strauß B, Wolfs M-J, Sappok A, Hoth M, et al. TRP expression pattern and the functional importance of TRPC3 in primary human T-cells. Biochim Biophys Acta BBA - Mol Cell Res (2011) 1813:412–23. doi: 10.1016/j.bbamcr.2010.12.022
41. Feske S. Immunodeficiency due to defects in store-operated calcium entry. Ann N Y Acad Sci (2011) 1238:74–90. doi: 10.1111/j.1749-6632.2011.06240.x
42. DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature (1984) 307:465–8. doi: 10.1038/307465a0
43. Panyi G, Possani L, de la Vega RC, Gaspar R, Varga Z. K+ Channel blockers: novel tools to inhibit T cell activation leading to specific immunosuppression. Curr Pharm Des (2006) 12:2199–220. doi: 10.2174/138161206777585120
44. Toldi G, Bajnok A, Dobi D, Kaposi A, Kovács L, Vásárhelyi B, et al. The effects of Kv1.3 and IKCa1 potassium channel inhibition on calcium influx of human peripheral T lymphocytes in rheumatoid arthritis. Immunobiology (2013) 218:311–6. doi: 10.1016/j.imbio.2012.05.013
45. Chauhan A, Sun Y, Sukumaran P, Zangbede FOQ, Jondle CN, Sharma A, et al. M1 macrophage polarization is dependent on TRPC1-mediated calcium entry. iScience (2018) 8:85–102. doi: 10.1016/j.isci.2018.09.014
46. Serafini N, Dahdah A, Barbet G, Demion M, Attout T, Gautier G, et al. The TRPM4 channel controls monocyte and macrophage, but not neutrophil, function for survival in sepsis. J Immunol (2012) 189:3689–99. doi: 10.4049/jimmunol.1102969
47. Honore P, Donnelly-Roberts D, Namovic M, Zhong C, Wade C, Chandran P, et al. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1αβ knockout mice. Behav Brain Res (2009) 204:77–81. doi: 10.1016/j.bbr.2009.05.018
48. Bhardwaj RS, Zotz C, Roth J, Goebeler M, Mahnke K, Falk M, et al. The calcium-binding proteins MRP8 and MRP14 form a membrane-associated heterodimer in a subset of monocytes/macrophages present in acute but absent in chronic inflammatory lesions. Eur J Immunol (1992) 22:1891–7. doi: 10.1002/eji.1830220732
49. Jinquan T, Vorum H, Larsen CG, Madsen P, Rasmussen HH, Gesser B, et al. Psoriasin: A novel chemotactic protein. J Invest Dermatol (1996) 107:5–10. doi: 10.1111/1523-1747.ep12294284
50. Lötzer K, Spanbroek R, Hildner M, Urbach A, Heller R, Bretschneider E, et al. Differential leukotriene receptor expression and calcium responses in endothelial cells and macrophages indicate 5-lipoxygenase–dependent circuits of inflammation and atherogenesis. Arterioscler Thromb Vasc Biol (2003) 23:e32–6. doi: 10.1161/01.ATV.0000082690.23131.CB
51. Mikulski Z, Hartmann P, Jositsch G, Zasłona Z, Lips KS, Pfeil U, et al. Nicotinic receptors on rat alveolar macrophages dampen ATP-induced increase in cytosolic calcium concentration. Respir Res (2010) 11:133. doi: 10.1186/1465-9921-11-133
52. Hsuan SL, Kannan MS, Jeyaseelan S, Prakash YS, Malazdrewich C, Abrahamsen MS, et al. Pasteurella hemolytica leukotoxin and endotoxin induced cytokine gene expression in bovine alveolar macrophages requires NF-kappaB activation and calcium elevation. Microb Pathog (1999) 26:263–73. doi: 10.1006/mpat.1998.0271
53. Dewin DR, Catusse J, Gompels UA. Identification and characterization of U83A viral chemokine, a broad and potent β-chemokine agonist for human CCRs with unique selectivity and inhibition by spliced isoform. J Immunol (2006) 176:544–56. doi: 10.4049/jimmunol.176.1.544
54. Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren’s syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol (2008) 181:2898–906. doi: 10.4049/jimmunol.181.4.2898
55. Martin L, Pingle SC, Hallam DM, Rybak LP, Ramkumar V. Activation of the adenosine A3 receptor in RAW 264.7 cells inhibits lipopolysaccharide-stimulated tumor necrosis factor-α Release by reducing calcium-dependent activation of nuclear factor-κB and extracellular signal-regulated kinase 1/2. J Pharmacol Exp Ther (2006) 316:71–8. doi: 10.1124/jpet.105.091868
56. Triggiani M, Gentile M, Secondo A, Granata F, Oriente A, Taglialatela M, et al. Histamine induces exocytosis and IL-6 production from human lung macrophages through interaction with H1 receptors1. J Immunol (2001) 166:4083–91. doi: 10.4049/jimmunol.166.6.4083
57. Shinkai A, Yoshisue H, Koike M, Shoji E, Nakagawa S, Saito A, et al. A novel human CC chemokine, eotaxin-3, which is expressed in IL-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J Immunol Baltim Md 1950 (1999) 163:1602–10. doi: 10.4049/jimmunol.163.3.1602
58. Schuerer-Maly CC, Eckmann L, Kagnoff MF, Falco MT, Maly FE. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology (1994) 81:85–91.
59. Thivierge M, Parent JL, Stankova J, Rola-Pleszczynski M. Modulation of formyl peptide receptor expression by IL-10 in human monocytes and neutrophils. J Immunol Baltim Md 1950 (1999) 162:3590–5. doi: 10.4049/jimmunol.162.6.3590
60. Locati M, Zhou D, Luini W, Evangelista V, Mantovani A, Sozzani S. Rapid induction of arachidonic acid release by monocyte chemotactic protein-1 and related chemokines. Role of Ca2+ influx, synergism with platelet-activating factor and significance for chemotaxis. J Biol Chem (1994) 269:4746–53. doi: 10.1016/S0021-9258(17)37607-X
61. Al-Aoukaty A, Rolstad B, Giaid A, Maghazachi AA. MIP-3alpha, MIP-3beta and fractalkine induce the locomotion and the mobilization of intracellular calcium, and activate the heterotrimeric G proteins in human natural killer cells. Immunology (1998) 95:618–24. doi: 10.1046/j.1365-2567.1998.00603.x
62. Proost P, Meester ID, Schols D, Struyf S, Lambeir A-M, Wuyts A, et al. Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV: CONVERSION OF RANTES INTO A POTENT INHIBITOR OF MONOCYTE CHEMOTAXIS AND HIV-1-INFECTION *. J Biol Chem (1998) 273:7222–7. doi: 10.1074/jbc.273.13.7222
63. Struyf S, De Meester I, Scharpé S, Lenaerts J-P, Menten P, Wang JM, et al. Natural truncation of RANTES abolishes signaling through the CC chemokine receptors CCR1 and CCR3, impairs its chemotactic potency and generates a CC chemokine inhibitor. Eur J Immunol (1998) 28:1262–71. doi: 10.1002/(SICI)1521-4141(199804)28:04<1262::AID-IMMU1262>3.0.CO;2-G
64. Wuyts A, D’Haese A, Cremers V, Menten P, Lenaerts JP, De Loof A, et al. NH2- and COOH-terminal truncations of murine granulocyte chemotactic protein-2 augment the in vitro and in vivo neutrophil chemotactic potency. J Immunol Baltim Md 1950 (1999) 163:6155–63.
65. Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4 *. J Biol Chem (1997) 272:15036–42. doi: 10.1074/jbc.272.23.15036
66. Proudfoot AEI. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol (2002) 2:106–15. doi: 10.1038/nri722
67. Salentin R, Gemsa D, Sprenger H, Kaufmann A. Chemokine receptor expression and chemotactic responsiveness of human monocytes after influenza A virus infection. J Leukoc Biol (2003) 74:252–9. doi: 10.1189/jlb.1102565
68. Berger EA, Murphy PM, Farber JM. CHEMOKINE RECEPTORS AS HIV-1 CORECEPTORS: roles in viral entry, tropism, and disease. Annu Rev Immunol (1999) 17:657–700. doi: 10.1146/annurev.immunol.17.1.657
69. Schols D, Struyf S, Damme JV, Esté JA, Henson G, Clercq ED. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med (1997) 186:1383–8. doi: 10.1084/jem.186.8.1383
70. Lusso P. HIV and the chemokine system: 10 years later. EMBO J (2006) 25:447–56. doi: 10.1038/sj.emboj.7600947
71. Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science (1995) 270:1811–5. doi: 10.1126/science.270.5243.1811
72. Taguchi K, Okada A, Kitamura H, Yasui T, Naiki T, Hamamoto S, et al. Colony-stimulating factor-1 signaling suppresses renal crystal formation. J Am Soc Nephrol (2014) 25:1680. doi: 10.1681/ASN.2013060675
73. Viedt C, Dechend R, Fei J, Hänsch GM, Kreuzer J, Orth SR. MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-κB and activating protein-1. J Am Soc Nephrol (2002) 13:1534. doi: 10.1097/01.ASN.0000015609.31253.7F
74. Betters P, RN DM. Use of flow cytometry in clinical practice. J Adv Pract Oncol (2015) 6:437. doi: 10.6004/jadpro.2015.6.5.4
75. Gauduchon V, Werner S, Prévost G, Monteil H, Colin DA. Flow cytometric determination of panton-valentine leucocidin S component binding. Infect Immun (2001) 69:2390–5. doi: 10.1128/IAI.69.4.2390-2395.2001
76. Kirchhoff K, Weinmann O, Zwirner J, Begemann G, Gotze O, Kapp A, et al. Detection of anaphylatoxin receptors on CD83+ dendritic cells derived from human skin. Immunology (2001) 103:210–7. doi: 10.1046/j.1365-2567.2001.01197.x
77. Princen K, Hatse S, Vermeire K, De Clercq E, Schols D. Evaluation of SDF-1/CXCR4-induced Ca2+ signaling by fluorometric imaging plate reader (FLIPR) and flow cytometry. Cytometry (2003) 51A:35–45. doi: 10.1002/cyto.a.10008
78. Heinemann A, Ofner M, Amann R, Peskar BA. A novel assay to measure the calcium flux in human basophils: effects of chemokines and nerve growth factor. Pharmacology (2003) 67:49–54. doi: 10.1159/000066786
79. Nishizaki Y, Oyama Y, Sakai Y, Hirama S, Tomita K, Nakao H, et al. PbCl2-induced hyperpolarization of rat thymocytes: Involvement of charybdotoxin-sensitive K+ channels. Environ Toxicol (2003) 18:321–6. doi: 10.1002/tox.10132
80. Lamoureux J, Stankova J, Rolapleszczynski M. Leukotriene D4 enhances immunoglobulin production in CD40-activated human B lymphocytes. J Allergy Clin Immunol (2006) 117:924–30. doi: 10.1016/j.jaci.2005.12.1329
81. Ceballos A, Sabatté J, Nahmod K, Martínez D, Salamone G, Vermeulen M, et al. Sphingosylphosphorylcholine activates dendritic cells, stimulating the production of interleukin-12. Immunology (2007) 121:328–36. doi: 10.1111/j.1365-2567.2007.02578.x
82. Chen Y-L, Chen Y-S, Lin H-H, Chan C-W, Chen S-C, Chen C-H. Immunostimulatory flagellin from Burkholderia pseudomallei effects on an increase in the intracellular calcium concentration and up-regulation of TNF-α by mononuclear cells. Microbiol Immunol (2007) 51:81–6. doi: 10.1111/j.1348-0421.2007.tb03893.x
83. Orbán C, Bajnok A, Vásárhelyi B, Tulassay T, Toldi G. Different calcium influx characteristics upon Kv1.3 and IKCa1 potassium channel inhibition in T helper subsets: Calcium Influx Kinetics in T Helper Subsets. Cytometry A (2014) 85:636–41. doi: 10.1002/cyto.a.22479
84. Gutzmer R, Langer K, Lisewski M, Mommert S, Rieckborn D, Kapp A, et al. Expression and function of histamine receptors 1 and 2 on human monocyte-derived dendritic cells. J Allergy Clin Immunol (2002) 109:524–31. doi: 10.1067/mai.2002.121944
85. Sun C, Jiang M, Zhang L, Yang J, Zhang G, Du B, et al. Cycloastragenol mediates activation and proliferation suppression in concanavalin A-induced mouse lymphocyte pan-activation model. Immunopharmacol Immunotoxicol (2017) 39:131–9. doi: 10.1080/08923973.2017.1300170
86. Tran HTT, Herz C, Ruf P, Stetter R, Lamy E. Human T2R38 bitter taste receptor expression in resting and activated lymphocytes. Front Immunol (2018) 9:2949. doi: 10.3389/fimmu.2018.02949
87. Boltz RC“Dutch”, Sirotina A, Blake T, Kath G, Uhrig B, McKeel J, et al. A disposable-chamber temperature-regulation system for the study of intracellular calcium levels in single live T cells using fluorescence digital-imaging microscopy. Cytometry (1994) 17:128–34. doi: 10.1002/cyto.990170204
88. Bates PJ, Ralston NV, Vuk-Pavlovic Z, Rohrbach MS. Calcium influx is required for tannin-mediated arachidonic acid release from alveolar macrophages. Am J Physiol-Lung Cell Mol Physiol (1995) 268:L33–40. doi: 10.1152/ajplung.1995.268.1.L33
89. Tárnok A, Dörger M, Berg I, Gercken G, Schlüter T. Rapid screening of possible cytotoxic effects of particulate air pollutants by measurement of changes in cytoplasmic free calcium, cytosolic pH, and plasma membrane potential in alveolar macrophages by flow cytometry. Cytometry (2001) 43:204–10. doi: 10.1002/1097-0320(20010301)43:3<204::AID-CYTO1051>3.0.CO;2-Z
90. Si M-S, Reitz BA, Borie DC. Effects of the kinase inhibitor CGP41251 (PKC 412) on lymphocyte activation and TNF-α production. Int Immunopharmacol (2005) 5:1141–9. doi: 10.1016/j.intimp.2005.02.012
91. Toldi G, Folyovich A, Simon Z, Zsiga K, Kaposi A, Mészáros G, et al. Lymphocyte calcium influx kinetics in multiple sclerosis treated without or with interferon beta. J Neuroimmunol (2011) 237:80–6. doi: 10.1016/j.jneuroim.2011.06.008
92. Orbán C, Szabó D, Bajnok A, Vásárhelyi B, Tulassay T, Arató A, et al. Altered calcium influx of peripheral Th2 cells in pediatric Crohn’s disease: infliximab may normalize activation patterns. Oncotarget (2016) 7:44966–74. doi: 10.18632/oncotarget.10036
93. Toldi G, Legány N, Ocsovszki I, Balog A. Calcium influx kinetics and the characteristics of potassium channels in peripheral T lymphocytes in systemic sclerosis. Pathobiology (2020) 87:311–6. doi: 10.1159/000509674
94. Novak EJ, Rabinovitch PS. Improved sensitivity in flow cytometric intracellular ionized calcium measurement using fluo-3/Fura Red fluorescence ratios. Cytometry (1994) 17:135–41. doi: 10.1002/cyto.990170205
95. Wendt ER, Ferry H, Greaves DR, Keshav S. Ratiometric analysis of fura red by flow cytometry: a technique for monitoring intracellular calcium flux in primary cell subsets. PLoS One (2015) 10:e0119532. doi: 10.1371/journal.pone.0119532
96. The evolution of spectral flow cytometry - Nolan - 2022 - Cytometry Part A - Wiley Online Library (Accessed 8 September 2023).
97. Panel Design and Optimization for High-Dimensional Immunophenotyping Assays Using Spectral Flow Cytometry - Ferrer-Font - 2020 - Current Protocols in Cytometry - Wiley Online Library (Accessed 8 September 2023).
98. Assis LH de P, Dorighello G de G, de Oliveira HCF. Pro-inflammatory polarization of macrophages is associated with reduced endoplasmic reticulum-mitochondria interaction. Biochem Biophys Res Commun (2022) 606:61–7. doi: 10.1016/j.bbrc.2022.03.086
99. Seegren PV, Harper LR, Downs TK, Zhao X-Y, Viswanathan SB, Stremska ME, et al. Reduced mitochondrial calcium uptake in macrophages is a major driver of inflammaging. Nat Aging (2023) 3:796–812. doi: 10.1038/s43587-023-00436-8
Keywords: flow cytometry, B cells, T cells, Ca2+ signaling, kinetics, immune cells, inflammation
Citation: Brun C, Chalet L, Moulin F, Bochaton T, Ducreux S, Paillard M and Crola Da Silva C (2023) A bibliometric analysis: Ca2+ fluxes and inflammatory phenotyping by flow cytometry in peripheral blood mononuclear cells. Front. Immunol. 14:1272809. doi: 10.3389/fimmu.2023.1272809
Received: 04 August 2023; Accepted: 02 October 2023;
Published: 13 October 2023.
Edited by:
Jeffrey John Bajramovic, Utrecht University, NetherlandsReviewed by:
Luiz Henrique Agra Cavalcante-Silva, Federal University of Paraíba, BrazilKatherina Psarra, Evaggelismos General Hospital, Greece
Copyright © 2023 Brun, Chalet, Moulin, Bochaton, Ducreux, Paillard and Crola Da Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claire Crola Da Silva, claire.crola-da-silva@univ-lyon1.fr; Melanie Paillard, melanie.paillard@univ-lyon1.fr; Sylvie Ducreux, sylvie.ducreux@univ-lyon1.fr
†These authors have contributed equally to this work and share last authorship
 Camille Brun
Camille Brun Lucie Chalet
Lucie Chalet Florentin Moulin1
Florentin Moulin1 Thomas Bochaton
Thomas Bochaton Sylvie Ducreux
Sylvie Ducreux Melanie Paillard
Melanie Paillard Claire Crola Da Silva
Claire Crola Da Silva