Abstract
The crystal structure of the Sb6O13 oxide, exhibiting a defect pyrochlore crystal structure with atomic vacancies, has been studied using a complete set of state-of-the-art techniques. The degree of antimony disproportionation in Sb3+ and Sb5+ valence states has been directly determined around 36% and 64%, respectively, using X-ray absorption near edge structure (XANES). These findings are in excellent agreement with our Rietveld analysis of synchrotron X-ray (SXRD) and neutron powder diffraction (NPD) results. Moreover, the highly distorted Sb3+ coordination due to its lone electron pair has been critically revisited. The bonding distances and coordination of Sb3+ and Sb5+ species closely agree with an extensive dynamic and crystallographic determination using the Extended X-ray Absorption Fine Structure (EXAFS) technique. Most importantly, the specific local disorder of the two distinctive Sb ions has been crosschecked monitoring their unusual Debye–Waller factors.
Similar content being viewed by others
Introduction
Despite significant progress reported in the technologies associated with the design of more effective batteries, the most quickly growing research field due to high environmental requirements and societal needs for effective energy storage systems, the exploratory work of alternative materials is still an active field. Besides more conventional materials for electrodes, Sb-derived oxides have been identified as appealing candidates to be used as battery anodes, for both Na and Li-ion batteries1,2,3,4.
Among Sb oxides, those exhibiting a pyrochlore-like structure (Sb2O3, Sb2O4, Sb2O5, Sb6O13)5,6,7 are particularly appealing, given the peculiarities of this structural type. The broad family of pyrochlore oxides, with the general formula A2B2O6O′(space group: \(Fd\overline{3}m\), Z = 8) displays an incomparable flexibility, concerning cationic substitutions, atomic vacancies, structural defects, and related superstructures, accounting for the wide panoply of physical properties and applications8, including thermal, electrical, and magnetic properties. They have also shown high resistance to radiation damage and temperature, and improved catalytic effects on water splitting9,10,11,12. Lately, a wide variety of pyrochlores have been the target of renewed attention due to their promising relevance in the field of fast ion conductors in Li-batteries13,14,15,16,17,18,19.
Many Sb-oxides present the so-called “defect pyrochlore structures”8, where the A and/or O′ atoms are absent or have a site occupation factor (SOF) lower than 1; an end-member formula AB2O6 is thus possible. Such a deficiency is intimately correlated with the fact that cation and anion migration within the solid is rather energetically feasible. By contrast, the vacancies in the B and O positions are rarely observed and, consequently, the (B2O6) framework (constituted by corner-sharing BO6 octahedra) of the pyrochlore structure is fairly rigid and stable. Hence, the free space of the above (B2O6) framework can be filled with a second framework related to the (A2O′) units, or with separate individual ions and/or H2O molecules.
Amid Sb-containing pyrochlore oxides with defective structure, Sb6O13 is a paradigmatic example, exhibiting half A and O′ sublattices, as derived from the crystallographic formula Sb′(Sb2)O6O′0.5. It therefore contains, nominally, Sb3+ ions at the A sublattice and Sb5+ ions at the octahedral B2O6 network. Pioneering preliminary structural studies carried out on Sb6O13 oxide, particularly based on laboratory XRD data20 allowed the determination of the main structural and displacement parameters21,22. An early X-ray absorption study on the Sb-based pyrochlore highlighted the importance of an approach based on a combination of diffraction and X-ray absorption techniques23.
Based on state-of-the-art techniques, this work reports on a more exhaustive complementary structural and chemical analysis using a combination of neutron and synchrotron X-ray powder diffraction (NPD and SXRD, respectively) together with Extended X-ray Absorption Fine Structure (EXAFS) and X-ray Absorption Near-Edge Structure (XANES) of Sb6O13 oxide. We thus accessed in a direct experimental way to a rather precise short- and long-range structural and dynamic description of the Sb6O13 oxide. Our findings show that both distinctive Sb species have very similar bond distances at short- and large-range; however, their dynamics are rather contrasting, evidencing large directional Debye–Waller factors (DW) characteristic of Sb3+. Such a study deepens the knowledge of their structural features, thus paving the way to a wide range of applications in batteries and catalysis.
Results and discussion
Long-range order structural determinations
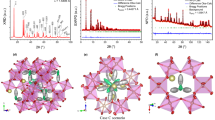
Well-crystalized Sb6O13 samples were obtained from the topotactic thermal decomposition of antimonic acid, as described in Methods. The structural features of this pyrochlore oxide with nominal crystallographic formula Sb3+Sb5+2O6O0.5 have been investigated with short- and long-range structural techniques, together with local chemical analysis. After revealing its pyrochlore nature with a prompt peak indexing over a laboratory XRD pattern, displayed in Fig. 1a, a more exhaustive structural analysis was carried out in the \(Fd\overline{3}m\) (# 227) space group, origin choice # 2, as previously reported for this material22, by means of extensive combined Rietveld refinement from SXRD and NPD diffraction data. Patterns obtained with both techniques established sharp diffraction peaks, characteristic of a cubic pyrochlore with a = 10.30653(11) Å, as displayed together with the refinement results in Fig. 1b,c, presented with more resolution in Supplementary Fig. S1. The basic (B2O6) covalent pyrochlore framework was defined with Sb atoms at 16d Wyckoff sites (½,½,½) and O at 48f. (x,1/8,1/8) positions. In order to locate the Sb3+ (Sb′) and O2− (O′) ions of the (Sb′2O′) sub-lattice in the cell, several attempts were carried out, including the use of Fourier difference density maps. The initial consideration of 16c positions for Sb′ and 8a sites for O′ (as usual in standard A2B2O7 pyrochlores) derived into large reliability factors, and unrealistically high Debye–Waller equivalent isotropic displacement factors (Ueq), of 0.0882(7) Å2 for the heavy Sb′ (about 9.6 times higher than that of Sb5+ atom) and 0.101(5) Å2 for O′, more than 6 times higher than that of O atom. Representations of this initially proposed structure are depicted in Fig. 1d,e. Later, a Fourier difference density map performed from NPD data, collected at room temperature (RT), led to negative scattering density at 8a position and a positive density dispersed in its vicinity, as shown in Supplementary Fig. S2. This, together with a similar inconsistency found at the Sb′ 16c site, suggested a wrong assignment of O′ and Sb′ atoms at those particular locations. Taking these results into account, a more thorough analysis, assisted by the Montecarlo simulated annealing technique, was performed from the NPD data. That led to our new proposed structure, where the Sb′ and O′ atoms differ in their Wyckoff sites from the previous model. This novel scheme was then employed for a final Rietveld refinement, for what the NPD together with SXRD data, also collected at RT, were combined. For comparison between the former and the new structure, the Rietveld plots from SXRD and NPD data are exhibited in Supplementary Fig. S1. The comparison of the equivalent isotropic displacement and Rietveld agreement factors is displayed in Supplementary Table S1.
Diffraction patterns and initial structural representation of the Sb6O13. (a) Laboratory XRD diagram of Sb′Sb2O6O′ (Cu Kα radiation) with peaks indexed in a face-centered cubic unit cell with a = 10.3065(1) Å. (b and c) Rietveld plots after combined refinement from SXRD and NPD data. Experimental (red crosses), theoretical (solid black line), and difference (solid blue line at the bottom) (b) SXRD and (c) NPD patterns, with Bragg reflection positions marked by vertical green bars. (d and e) Different views of the firstly proposed pyrochlore structure; the size of the atoms is proportional to the ionic radii. Caption color reference at the bottom of this figure is common for both panels. (d) Scheme of the unit cell approximately along the \(\left[ {\overline{1}01} \right]\) direction. Yellow (111) and green (222) crystallographic planes are displayed, whose coplanar atoms contribute for the 111 and 222 peaks in (a), respectively. (e) Two different views of 1/8 of a unit cell, where the four crystallographically independent atoms are highlighted. Here, 8a (O′, green) and 16c (Sb′, blue) sites are only half occupied, given by their SOF’s of ½ for both cases.
Summarizing, the optimal partial occupancy of the 96g and 32e Wyckoff sites by Sb′ and O′, respectively, are recapitulated in Table 1. The equivalent isotropic displacement factors obtained for Sb′ and O′ atoms in the new 96g and 32e positions (0.035(4) and 0.023(5) Å2, respectively) were significantly lower than those obtained in the previous model refinement, much more consistent with the expected magnitude. Though somewhat greater than the equivalent parameters of Sb (8.77(11) × 10−3 Å2) and O (1.53(6) × 10−2 Å2), they are reasonable, considering their higher multiplicity, and thus, reduced occupancy and their location within the wide cavities created by the (Sb2O6) framework, from which a certain diffusivity is expected for both Sb′ (Sb3+) and O′ (O2–) atoms across its channels.
For the 16c for Sb′ and 8a for O′ Wyckoff sites, the latter appears to be tetrahedrally coordinated by the Sb′ atoms at 2.2314(2) Å, with the Sb′‒O′‒Sb′ tetrahedral angle of 109.5°; only 1 out of 2 Sb′ and O′ atoms are present, as ½ of the 16c and 8a sites are statistically occupied. The new proposed 96g and 32e Wyckoff sites yield a range of distances and angles, all close to each other. Although Sb′‒O′ distances range from 2.164(12) to 2.771(9) Å, the new SOF’s (1/12 for Sb′ and 1/8 for O′) of both species suggest that only the realistic bonding distances (i.e. those approaching the ionic radii sum of 2.16 Å) would take place, being in this scenario the shortest one; Sb′‒O′‒Sb′ angles vary in the range of 85.4(6)° to 119.4(7)°. The mean O′ coordination seems to remain, as the Sb′ possible sites are spread around the 16c as the O′ does for the 8a site. Effectively, these wide distances and angles variation observed in the Sb6O13 structure is indicative of the great motility of these two species. Consequently, the (Sb′2O′) sub-lattice is essentially reduced to a random distribution of V-shaped Sb′–O′–Sb′ groups, constituted by two pairs of Sb′–O′ bondings (2.164(12) Å in length), in a tetrahedral distribution with characteristic angle 111.1(7)°, as displayed in a snapshot of the structure in Fig. 2c. It is worth noting that the Sb′ stereochemically active lone electron pair would be liable for the atom displacement to a higher multiplicity Wyckoff site. This prompts the coordination of Sb3+ species to three closer non-bonding O atoms, one at 2.242(9) Å and two at 2.457(9) Å, together with a bonding O′ at 2.164(12) Å, leaving Sb′ with four-fold coordination and its lone pair directed to its former 16c position; Fig. 2e and Supplementary Fig. S2 provides schematic descriptions. Also, an animated representation of a single (Sb′2O′) unit in its (Sb2O6) framework cavity from the final refined structure is displayed in Supplementary Fig. S3.
This final refined structure for the Sb6O13 oxide is strongly endorsed by one of our recent reports, where some of our group revisited the general structural data of a pyrochlore family with a similar (B2O6) framework24. Certainly, the shift of the O′ atoms to the 32e (x,x,x) sites, concerning the lower-multiplicity 8a (1/8,1/8,1/8) position, has been confirmed as a recurrent peculiarity throughout the whole pyrochlore family24. Indeed, in Sb6O13, the distribution of Sb3+ ions at 96g instead of 16c Wyckoff positions is presumably due to the lone electron pair and its stereochemical effect, that would displace, by repulsion forces, the Sb′ atom about 0.353(9) Å from the (0,0,0) position to an adjacent 96g (x,x,z) site, with six-times greater multiplicity. The constrained SOF’s values, chosen from previous reports22, were selected to get a coherent and electrically neutral formula, and they stand in reasonable agreement with those obtained from XANES, see more details below. Furthermore, we confirm that any attempt to add H+ atoms systematically failed, proving the absence of water in the material, which is consistent with the results reported from pioneering analyses performed by Stewart et al.21. After the Rietveld combined analysis, an utter concordance between observed and calculated neutron and synchrotron X-ray diffraction profiles was achieved, as displayed in Fig. 1b,c. A Scherrer25,26 domain size estimation from SXRD data gives an apparent crystallite size of 40.02(6) nm. For Scherrer’s apparent domain size determination, instrumental broadening was deconvoluted, see Methods for more details.
The main interatomic distances and angles from the new structure are summarized in Table 2. Different views of the final crystal structure are displayed in Fig. 2.
Views of the Sb6O13 pyrochlore final structure. Atoms in all six panels are presented as anisotropic displacement ellipsoids at a 95% probability level. Caption color reference at the bottom of this figure is common to all panels. (a) Scheme of the unit cell approximately along the \(\left[ {\overline{1}01} \right]\) direction. Yellow (111) and green (222) crystallographic planes are displayed. (b) Two views of 1/8 of a unit cell, where the four crystallographically independent atoms are highlighted. Statistically, only 1/12 Sb′ (blue, 96g Wyckoff site) and 1/8 O′ (green, 32e site) are present. (c) Representation of a snapshot of the crystal approximately along the \(\left[ {01\overline{1}} \right]\) direction: the covalent framework made by the (Sb5+2O6) corner-sharing octahedra consists of 32e (Sb) and 48f. (O) fully occupied sites, while the (Sb′2O′) sub-lattice reduces essentially to a random distribution of oxygen atom center V-shaped groups constituted by two pairs of Sb′–O′ bondings (2.164(12) Å in length), in a 111.1(7)° angled tetrahedral distribution. (d) Close up of a single cavity wherein the Sb′ and O′ are distributed at 96g and 32e Wyckoff positions with SOF’s of 1/12 and 1/8, respectively. (e) Coordination of Sb′ (Sb3+) species to three O and one O′ oxygen atoms, with its lone electron pair directed to the center of the cavity (16c Wyckoff site). The yellow tetrahedron represents the electronic geometry of a single element of the (Sb′2O′) unit.
Structural and chemical short-range order studies
For the structural and chemical short-range order studies, we focused on XAFS (X-ray Absorption Fine Structure) in Sb6O13 and other reference compounds (i.e. Sb, Sb2O3, Sb2O4, and FeSbO4), which we studied under the same equipment operative conditions. Such model samples have been particularly useful to unveil the local chemical environment and establish a structural comparison with our target material Sb6O13. We start our discussion by the X-ray Absorption Near-Edge Structure (XANES) at the Sb K-edge energy, and then the extended region of the XAFS spectra will be treated. In Fig. 3a, the room temperature XANES spectra (vertically shifted for clarity) for Sb6O13 is displayed; besides some XANES spectra of model compounds with nominal valence states including Sb foil (for Sb0), Sb2O3 (for Sb3+), FeSbO4 (for Sb5+), and Sb2O4, being corrected in energy by the absorption edge of Sb0 with energy position at the Sb K-edge (30,491 eV). Effectively, the energy position of the K-edge, even if it concerns to an Sb inner level (or core level), is extremely sensitive to the chemical environment of the antimony, since the binding energy of these bound electrons increases with the valence. Each XANES spectrum contains particular features, which denotes that a structural distortion is distinctive for both, the reference compounds and the target Sb6O13 sample. It is worth mentioning, in agreement with the scarce previous results reported so far, that all the XANES spectra show the absence of a pre-edge peak structure.
In order to extract chemical information from the Sb6O13 XANES spectra, we performed a Linear Combination Fitting (LCF) taking into account the Sb2O3 and FeSbO4 spectra, which can be considered as model compounds exclusively containing Sb+3 and Sb+5 ions, respectively, see Fig. 4a. Indeed, from the XANES spectra of the Sb6O13 oxide the determined composition corresponds to 36(1) at% and 64(1) at% for Sb3+ and Sb5+, respectively. These findings agree well with the expected nominal concentrations derived from a stoichiometric crystallochemical relationship, as follows: Sb2O3 + 2Sb2O5 → Sb6O13. Going along with the same procedure, as shown in Fig. 4b, the XANES spectrum of Sb2O4 compound has been precisely reproduced to 51(1) at% and 49(1) at% contributions of the Sb2O3 and FeSbO4 XANES spectra, respectively. The Sb2O4 contains a stoichiometric mixture of Sb3+ and Sb5+ in an atomic ratio Sb3+/Sb5+ = 50/50. The absorption edge is sensitive to the oxidation state of the antimony element; Fig. 4c shows the increase in the value of the absorption edge for different antimony compounds, starting from Sb foil (Sb0) up to FeSbO4 (Sb5+). It is worth noting that the XANES spectra at Sb K-edge energy of the Sb compounds Sb2O4 and FeSbO4 have only been partially reported27, hence its description in the present work is also valuable as a reference.
Valence state analysis from XANES technique. Linear Combination Fitting (LCF) showing the percentage of Sb3+ and Sb5+ in (a) Sb6O13 and (b) Sb2O4 pyrochlores. (c) The variation in the absorption edge as a function of the antimony valence state for all the compounds investigated in this work. The dotted line is only a guide for the eye.
At this point, we start to evaluate quantitatively the short-range order using the extended part of the XAFS spectra (EXAFS). The EXAFS oscillations (vertically shifted) for Sb6O13 and the model compounds Sb foil, Sb2O3, FeSbO4, and Sb2O4, together with their respective moduli of the Fourier transform in R space, are depicted in Fig. 3b,c, respectively. The short-range character of the structural data obtained from the EXAFS is based on the typical EXAFS equation, which is conventionally used as a model for fitting the experimental extended region of the XAFS data28,29, as follows:
where Γ represents each path taken by the photoelectron to the closer coordination shells containing the neighboring atoms of the absorber, the antimony, in this case. Therefore, the complete EXAFS oscillations extracted from the spectra are the convolution of all the paths under consideration, which involve the absorption processes locally around the absorber atoms, in a short-range regime. This is of the order of the detection limit of the technique (< 5 Å), i.e.:
RΓ is the mean distance between the absorber atom (or emitter) and its neighboring atoms, NΓ denotes the number of neighboring atoms within the shell, S02 is the amplitude reduction factor obtained from a previous calibration using a well-known standard of metallic antimony (in our case, S02 was 0.7802 for all the samples, as obtained from EXAFS fitting of Sb foil), σΓ2 concerns the Debye–Waller (DW) factor, which measures the mean square relative displacement, being linked to the atomic motion30. FΓ(k, RΓ), λ(k), and φΓ(k) are defined as backscattered amplitude, photoelectron mean free path, and phase shift, respectively, which are calculated using the FEFF-8 code. The EXAFS spectra were adjusted in the framework of the ARTEMIS software, which works within the FEFF’s multiple-scattering path expansion31.
Figure 5 represents the k3 weighted χ(k) EXAFS oscillations and their fittings (a) for the Sb6O13 and the model compounds (c), together with the respective moduli of the Fourier transform in R space (b) and (d). We will discuss in detail the EXAFS data obtained for each oxide compound. Although our target oxide here is Sb6O13, the analysis of some standards is indeed interesting since their XAFS data are scarce in literature and they provide robust bases to appropriately interpret the EXAFS spectra of the Sb6O13 oxide. It should be emphasized that the upper limit of independent variables imposed by the uncertainty principle, Nidp, depends on the wavenumber Δk and radial ΔR intervals employed during the data processing. For all the fittings, we have defined Δk = 12.5 Å−1 and ΔR = 3.3 Å, leading to a Nidp ≈ 2ΔkΔR/π ~ 26. The structural parameters for each shell including coordination number, bond distance, and Debye–Waller factor were taken as adjustable settings.
EXAFS analysis in Sb-based oxides. (a) k3-weighted EXAFS signals of Sb6O13 at Sb K-edge and (b) their corresponding Fourier transform magnitude. (c) k3-weighted χ(k) oscillations of model compounds (Sb, Sb2O3, FeSbO4, and Sb2O4) and (d) their respective Fourier moduli |χ(R)|. The open symbol represents the experimental point, while the solid black line is the best fit obtained.
We start the EXAFS analysis of the local structure in the antimony oxides with a mixed-valence state, for the case of Sb6O13 and Sb2O4. It should be noticed that all the Fourier transforms in Fig. 3c have a main peak at 1.52 Å followed by a second feature above 2.5 Å in R space (not corrected by photoelectron phase-shift), except the Sb metal, with a single peak around 2.65 Å. As seen from the diffraction results, the Sb6O13 oxide has two antimony atoms, Sb (Sb5+) and Sb′ (Sb3+), which are split out into different sites within the pyrochlore lattice. Indeed, the atomic distances for Sb‒O and Sb′‒O′ are 1.9624(5) and 2.164(12) Å, respectively. Therefore, two close main peaks should be detected in the Fourier transform |χ(R)|, following the quantification obtained from XANES data. Instead, a single feature in R space at 1.52 Å, similar to the Sb2O3 and FeSbO4 (with a single Sb valence, 3 + and 5 + , respectively), is noticeable for both Sb6O13 and Sb2O4. From the Eq. (1), if we consider similar R distances for paths composed by Sb5+‒O and Sb3+‒O′, then an effective path for the oxygen shell will be formed containing an average coordination number.
Using such an approach, the EXAFS fitting for Sb6O13 in Fig. 5a,b showed that the first oxygen shell \(\langle {\text{Sb}}\rangle - \langle {\text{O}}\rangle\) pair bond has an average coordination number of \(N_{{\langle {\text{Sb}}\rangle - \langle {\text{O}}\rangle }} = 5.7\left( 2 \right)\) with a distance of \(R_{{\langle {\text{Sb}}\rangle - \langle {\text{O}}\rangle }} = 1.957\left( 1 \right)\) Å, which in turn agrees with the Sb‒O and Sb′‒O′ bond distances determined from the diffraction results, in Table 3. The coordination number \(N_{{\langle {\text{Sb}}\rangle - \langle {\text{O}}\rangle }}\) has an intermediate value between 3 (distorted trigonal pyramidal, Sb3+) and 6 (octahedral, Sb5+), but very close to six and denoting that the EXAFS description of the oxygen coordination shell is mainly dependent on the Sb5+‒O pair bonds27. In Table 2, the distances 3.2‒4.1 Å concerns the non-bonding metallic-metallic pairs of antimony, which include the Sb‒Sb, Sb‒Sb′, and Sb′‒Sb′ pairs. Instead, the EXAFS fitting with a metallic-metallic pair revealed a threefold coordination with \(N_{{\langle {\text{Sb}}\rangle - \langle {\text{Sb}}\rangle }} \sim 2.8\left( 9 \right)\) and an average distance of \(R_{{\langle {\text{Sb}}\rangle - \langle {\text{Sb}}\rangle }} = 3.642\left( 1 \right)\) Å. This low coordination number value may be explained as a result of the spread in the pair bond distribution; however, the pairs Sb5+‒Sb5+ and Sb5+‒Sb3+ were observed, since the distance obtained from EXAFS (\(R_{{\langle {\text{Sb}}\rangle - \langle {\text{Sb}}\rangle }} = 3.642\left( 1 \right)\) Å) was very close to that obtained from the diffraction data (dSb‒Sb = 3.644‒3.661 Å). In the high-R space position, it can be observed an extra oxygen shell with a typical distance of dSb‒O = 4.4729(4) Å (summarized in Table 3). From the EXAFS, this radial distance was obtained as \(R_{{\langle {\text{Sb}}\rangle - \langle {\text{Sb}}\rangle }} = 4.476\left( 1 \right)\) Å, being coordinated by \(N_{{\langle {\text{Sb}}\rangle - \langle {\text{Sb}}\rangle }} = 5\left( 1 \right)\) oxygen anions, probably denoting the Sb5+‒O pair bonds.
Similar to the Sb6O13 mixed oxide, the α-Sb2O4 oxide has a distribution of Sb3+ and Sb5+ split out into two different positions within the room-condition orthorhombic Pna21 space group (C2v9 or Nº 33)32. The EXAFS spectrum of such an oxide was reported at Sb K-edge for its mineral form, known as stibiconite (cubic) and cervantite (orthorhombic)27. Here, we employed the orthorhombic phase as a reference for further comparison among XAFS and diffraction data. According to the diffraction data32, the Sb5+ ion has a nearest oxygen shell containing six anions separated by 1.92‒2.11 Å, while the Sb3+ ion possesses, in its nearest oxygen shell, four anions separated by distances of 2.01‒2.25 Å. Using the same approach for Sb6O13 XAFS data, the EXAFS fitting showed that the first shell \(\langle {\text{Sb}}\rangle - \langle {\text{O}}\rangle\) pair bond for Sb2O4 has \(N_{{\langle {\text{Sb}}\rangle - \langle {\text{O}}\rangle }} = 4.9\left( 2 \right)\), with a radial distance of \(R_{{\langle {\text{Sb}}\rangle - \langle {\text{O}}\rangle }} = 1.971\left( 1 \right)\) Å. Undoubtedly, it reflects the effect of the mixed-valence state through the coordination number. A second oxygen shell was fitted with \(N_{{\langle {\text{Sb}}\rangle - \langle {\text{O}}\rangle }} = 0.9\left( 9 \right)\) separated by a radial distance of \(R_{{\langle {\text{Sb}}\rangle - \langle {\text{O}}\rangle }} = 2.598\left( 1 \right)\) Å. From the diffraction data, this shell represents three Sb3+‒O pairs with a bonding distance between 2.53‒3.03 Å and, then, evidence for the occurrence of Sb3+ ions as detected by EXAFS spectra. In addition, the metallic-metallic shells were fitted using three scattering paths with radial distances RSb‒Sb at 3.410(1), 3.630(1), and 3.920(1) Å, which correspond to the coordination numbers NSb‒Sb of 1.3(5), 1.7(8), and 3.3(2), respectively. These scattering paths corroborate the diffraction data in view of the following pair distances: Sb3+‒Sb3+ (× 2) with 3.423 Å; Sb5+‒Sb5+ (× 2) with 3.620 Å; Sb5+‒Sb3+ (× 2) and Sb3+‒Sb3+ (× 2) with 3.83‒3.91 Å, respectively.
At this point, we have reinforced the individual structural study of Sb2O3 oxides by a comparative and systematic EXAFS research of Sb2O3 and FeSbO4 model compounds. Although their descriptions are already reported in literature27,33, here we deeply revisit their short-range structure using the EXAFS technique for both single-valent compounds. The EXAFS signal of Sb2O3 was adjusted using four neighboring shells of antimony, namely two Sb3+‒O and other two Sb3+‒Sb3+ bonds. Details on the numerical results are summarized in Table 3; a good agreement with previous EXAFS data of Sb2O3 was obtained27. This compound has an orthorhombic unit cell within the Pccn space group (D2h10 or Nº 56), in which Sb3+ ions are located at 8e Wyckoff position34,35. The main peak around ~ 1.55 Å in R distance (not corrected by photoelectron phase-shift) concerns the Sb3+‒O bond of RSb‒O = 1.978(1) Å, with a coordination number of NSb‒O = 4.0(2). The second main peak around ~ 3.38 Å in R concerns the Sb3+‒Sb3+ distance (RSb‒Sb = 3.631(1) Å) with NSb‒Sb = 1.9(4). For FeSbO4, we analyzed its first (oxygen) and third (metallic) shells. Such an oxide possesses a tetragonal unit cell belonging to the P42/mnm space group (D4h14 or Nº 136). The Sb5+ ions locate at 2a Wyckoff sites, half shared with Fe3+ cations. The most intense peak in the Fourier transforms denotes the first oxygen shell six-coordinated NSb‒O = 6.3(5) with a bond distance of RSb‒O ~ 1.977(1) Å. The second feature in R space is also assigned to the Sb5+‒Sb5+ pair (RSb‒Sb ~ 3.633 Å) in a threefold coordination, since NSb‒Sb = 3.3(4). In this example, we also considered two scattering paths with the Sb5+‒Fe3+ interatomic pairs due to the partial occupancy at this site, as follows: the first one at RSb‒Fe = 3.141(1) Å and the second one RSb‒Fe = 3.654(1) Å.
Although the EXAFS fittings were done without a priori distinction for the valence states of antimony, the presence of Sb3+ and Sb5+ can be inferred in the extended region of XAFS spectra by the Debye–Waller factor (\(\sigma_{\Gamma }^{2} = \langle \Delta u^{2} \rangle\)). Such a factor stands for the structural and vibrational disorder effects that damp the EXAFS signal30,36. Usually, this coefficient depicts distinct values as extracted from EXAFS and diffraction data. The main reason for that lies on the nature of the average defined for each situation: in EXAFS, such a value is obtained from the nearest coordination shells, while, in diffraction techniques, the average is performed by taking the whole crystal structure into account (3N vibrational modes in Debye’s model30,37). Therefore, the Debye–Waller extracted from EXAFS data shows a higher sensitivity to local disorder. The behavior of σΓ2 against the average valence state (those obtained from XANES data) is represented in Fig. 6a for the nearest oxygen shell \(\langle {\text{Sb}}\rangle - \langle {\text{O}}\rangle\). One may see an increase for Sb6O13 and Sb2O4 samples from ~ 2.7 × 10−3 to above 3.5 × 10−3 Å2, followed by a decrease for the FeSbO4 oxide. It means that a pair bond disorder of the mixed-valence states of antimony ions takes place. It is also important to emphasize that the ionic radii may trigger this disorder since Sb5+ has an ionic radius of 0.60 Å for sixfold coordination while Sb3+ has one of 0.76 Å for both 4- and sixfold coordination38. In the case of FeSbO4, the path regarding Sb‒Fe pair at RSb‒Fe = 3.654(1) Å, also has a disorder factor of the same magnitude than that of Sb6O13 in high order metallic shells, i.e. σΓ2 = 4.7 × 10−3 Å2, corroborating the increase in the Debye–Waller factor.
EXAFS parameters against the Sb valence state. Structural parameters extracted from the EXAFS fittings, particularly (a) Debye–Waller factor (σΓ2), (b) coordination number (NΓ), and (c) radial distribution (RΓ), for oxygen and metal shells, against the antimony valence state. In panel (c), the radial distances Sb5+‒O and Sb5+‒Sb5+ of Sb2O5 were extracted from Ref27.
The variation of the local environment of the nearest oxygen shell can also be probed using the coordination number, as shown in Fig. 6b. The progressive increase of NSb‒O from ~ 4 up to 6 represents a tendency for sixfold coordination expected for Sb5+-type cations. In the case of Sb2O4, the reported structural features from diffraction data establish two different sites for both Sb3+ (fourfold) and Sb5+ (sixfold) ions in a 50/50 proportion, leading to an average coordination number of ~ 5, which agrees with that observed by EXAFS fitting: NSb‒O ~ 4.9(2). For the Sb6O13 oxide, a sixfold coordination is observed either for Sb5+ at 16d Wyckoff sites and a four-fold for Sb3+ at 96g sites, which results in average coordination close to 6 (\(N_{{\langle {\text{Sb}}\rangle - \langle {\text{O}}\rangle }} \sim 5.7\left( 2 \right)\)), with \(\langle {\text{Sb}} - {\text{O}}\rangle\) average distances of 1.9‒2.2 Å. In the literature, EXAFS treatments at Sb L1-edge (4698 eV) were performed by Rockenberger et al.33 over Sb-doped tin oxide; the authors argued that the Sb3+ atoms are mainly distributed at surface sites (or grain boundaries) of Sb-based nanoparticles and, then, the first coordination shell Sb‒O is dominated by Sb5+ ions. Obviously, in nanoparticles with particle size below 10 nm, it will represent segregation for surface sites. For the mixed oxide Sb6O13, the previous fitting of the nearest oxygen shell was carried out with both valence states using pair bonds Sb3+‒O and Sb5+‒O, but with no reasonable data description. In this way, the presence of Sb3+ in Sb6O13 was also probed using the EXAFS parameters of higher-order metallic shells, for instance, Sb‒Sb shell at ~ 3.6 Å in the |χ(R)| FT spectra (without phase-shift correction). Figure 6 also contains the structural EXAFS parameters (σΓ2, NΓ, and RΓ) extracted from EXAFS fitting, where the DW factor for the pair Sb‒Sb in Sb6O13 exhibits an enlarged value (5(2) × 10−3 Å2) as compared to the Sb2O3 (1.1(9) × 10−3 Å2) and FeSbO4 (2.9(6) × 10−3 Å2) oxides, as a result of the disorder induced by the mixed-valence states of Sb3+ and Sb5+. The bond distance RΓ, in Fig. 6c, for the mixed-valence oxides, show similar values than those observed for Sb2O3, but slightly higher, meaning that the higher-order shells in Sb6O13 and Sb2O4 have Sb5+‒Sb3+ pairs, probably as a consequence of the electrostatic repulsion between both ions.
Conclusions
The complementary structural and dynamic study of Sb6O13 using long- and short-range diffraction techniques together with X-ray Absorption Fine Spectroscopy confirms that this particular Sb-oxide can be precisely described as a defect pyrochlore, defined in the \(Fd\overline{3}m\) space group. More importantly, Sb3+ and O′ ions, located in large multiplicity sites, ensures the flexibility and mobility of the Sb6O13 oxide, a frequent characteristic of pyrochlore oxides. Interestingly, our findings based on a comprehensive crystal structure analysis revealed that the degree of Sb disproportionation in the Sb3+xSb5+yO6O′ oxide is close to a ratio Sb3+/Sb5+ = 0.56, with x = 2.15 and y = 3.85 as the abundance of Sb3+ and Sb5+ in the unit cell, respectively. This is in agreement with the long-range structural results refined from SXRD and NPD data, yielding the nominal Sb3+/Sb5+ = 0.50 atomic ratio, with x = 2.0 and y = 4.0, with the obtaining of an electrically neutral structure and fairly good reliability factors. Based on X-ray Absorption analysis at Sb K-edge energy in Sb6O13 and other Sb-containing compounds like Sb2O3, FeSbO4, and Sb2O4, where Sb3+, Sb5+, or both cations are simultaneously found, we also unveiled unreported features that are worth describing to complete the knowledge of the appealing field of antimony oxides. Using the XANES technique, the valence state proportion Sb3+/Sb5+ was estimated by a Linear Combination Fitting for Sb2O4 and Sb6O13. Complementary, the EXAFS technique was valuable to locally infer the occupancy of antimony ions Sb3+ and Sb5+ into the crystalline structure of Sb6O13. The Debye–Waller factor was essential to unveil the presence of Sb3+ within the local structure, which means that EXAFS can be used to characterize the presence of Sb3+ within the channels of the pyrochlore structure.
Methods
Sample preparation
All the commercially available ReagentPlus or Analytical-grade reagents were purchased at Sigma Aldrich and Fisher Scientific.
The compound Sb6O13 has been synthesized in a two-step procedure, a soft-chemistry reaction followed by a thermal topotactic reaction. This topotactic synthesis pathway was previously described in the literature, with little variations in its thermal treatment39. It begins from the so-called Antimonic Acid (AA), also presenting a pyrochlore structure with the \(Fd\overline{3}m\) (# 227) space group. AA was primarily obtained by treatment of Sb2O3 (99.7% Alfa Aesar) in 31% H2O2 (Merk) stirred for 24 h at 70 °C. The white colloidal suspension was then centrifuged at 15,000 rpm for 10 min until most of the solid was deposed in the bottom. The white material was then dried in air at 80 °C for 48 h, and subsequently calcined in an open alumina crucible at 550 and 700 °C for 12 h each treatment, with intermediate and final grindings, following the reaction:
The contents of Sb and O in the AA precursor sample were determined by using the ICP-OES technique with a Perkin-Elmer 3300DV instrument after nitric acid digestion, and the obtained composition (53.31 wt% Sb and 46.43 wt% O) are in reasonable agreement with the theoretical values (53.39 wt% Sb and 46.61 wt% O).
Polycrystalline Sb6O13 was reground to a fine powder in an agate mortar and then initially investigated using X-ray powder diffraction. Laboratory XRD data for Sb6O13 were collected with a Bruker-AXS D8 Advance diffractometer (40 kV, 30 mA) (Germany) controlled by the DIFFRACTPLUS software, in Bragg–Brentano reflection geometry, with Cu Kα radiation (λ = 1.5418 Å). The SXRD pattern was collected in the MSPD high-angular resolution diffractometer at the CELLS‒ALBA facility, Barcelona (Spain), selecting an incident beam with a 28 keV energy, λ = 0.44271 Å, together with powdered Na2Ca3Al2F14 fluoride (NAC) as standard for determining the instrumental broadening. The high-angular resolution mode (MAD set-up) on the MSPD-diffractometer was utilized. The polycrystalline powder was contained in a glass capillary of 0.7 mm diameter, which was rotated during the acquisition time. NPD experiments were carried out in the D2B high-resolution powder diffractometer (λ = 1.5947(1) Å) at the Institut Laue-Langevin, in Grenoble (France). About 2 to 3 g of the sample was contained in a vanadium can. The full diffraction patterns were collected in 2 h of measurement time.
Rietveld refinement of Synchrotron and Neutron diffraction data
A combined Rietveld refinement20 from both SXRD and NPD data was carried out with the software FULLPROF40 (Grenoble, France, September-20, 2019 version). A relative pattern weight of 20/80, respectively, was considered. The adopted weight in favor of neutron diffraction data was given due to its absence of form factor and its unique sensitivity even for light elements like O, preferred for determining oxygen atomic displacement factors (ADPs). Even when SXRD offers far better counting statistics than the NPD technique, intermediate weighting combinations, from 80/20 to 20/80, result into unlikely displacement probabilities. The best Rietveld reliability factors and realistic ADPs were achieved for the 20/80 relationship, favoring the NPD data. For comparison, those attained for SXRD/NPD weightings of 50/50 and 20/80 are shown in Supplementary Table S2.
For the NPD data treatment, a pseudo-Voigt41 function with the asymmetry correction published by Berar and Baldinozzi42 was used for the simulation of the peak shape and its asymmetry correction, respectively. For the SXRD data refinement, the Thompson-Cox-Hastings43 pseudo-Voigt convoluted with axial divergence asymmetry and pseudo-Voigt was employed. An absorption correction coefficient of μR = 0.92 was determined and included in the refinement to account for the transmission and absorption of the X-rays through the irradiated cylindrical volume of the sample. For its determination, a packed fraction of 0.5 was adopted. The microstrains and domain size modules included in FULLPROF, the latter based on Scherrer’s equation, were used for an apparent crystallite size and generalized strain determination. The calculated apparent isotropic crystallite sized of 40.02(6) nm and average maximum strain of ε = 3.283(7) × 10−4 were obtained. The announced standard deviations for both measurements are indicative of their degree of anisotropy, and not error estimations. On the other hand, both SXRD and NPD backgrounds were approximated with 24-term Chebyshev refined polynomials. The bound coherent neutron scattering lengths used in the Rietveld refinement are internally tabulated in the program FULLPROF, and their values are 5.570 and 5.803 fm for Sb and O, respectively.
At the final stages of the Rietveld analysis, the individual atomic anisotropic displacement (“thermal”) parameters were successfully refined independently for each nonequivalent atom, that is to say for Sb, Sb′, O, and O′. Application of the corrections for surface roughness and preferred orientation did not improve the structural model of Sb6O13, so these corrections were not refined with FULLPROF.
The refinement converged with Bragg R-factors RB of 3.61% for SXRD and 2.34% for NPD, which are fairly low values, in agreement with the theoretical and experimental structural model. It is worth saying that RB intensity residuals are not affected by the background level, but profile R values Rp and Rwp, although commonly used, are not satisfactory from a statistical point of view. In practice, they depend on nonstructural effects such as background44. Being this particularity clarified, Rp, Rwp, and expected weighted profile factor Rexp are summarized at the bottom of Table 1.
X-ray Absorption Spectroscopy at the CLÆSS beamline of the ALBA synchrotron
The X-ray absorption process was performed by measuring the photon flux before and after the interaction with the sample as an energy function of the incoming photons. This well-established technique used in transmission or fluorescence mode provided an exact measurement of the X-ray absorption coefficient μ(E). The resulting absorption spectra were then characterized by one or more jumps (absorption edges), whose energy positions are element specific since they coincide with the energy of the corresponding atomic core level. Furthermore, as the X-ray transitions are controlled by the dipolar selection rules relating to well-defined atomic symmetry of the involved core hole and the final state angular momenta, the XANES spectra show a remarkable site-specific behavior, because they are sensibly affected by the strong spatial localization of the initial core–shell state.
The XAFS experiments presented in this work were performed using the extremely stable operation conditions of the CELLS‒ALBA Synchrotron, with electron energy and current in the ring of 3 GeV and 200 mA, respectively. At present, this installation operates in top-up, which keeps the electron current in the storage ring constant at typically 200 (± 0.5) mA, ensuring a constant heat load and, consequently, minimal thermal drifts of the associated optics of the beamline. The BL22-CLÆSS beamline provides access to the X-ray absorption technique and emission spectroscopies45. The beamline is equipped with a Wiggler of a total length of 1 m, where 12 periods of 80 mm each are installed. The incoming energy range is 2.4–63.2 keV. The XAFS experiments are performed using the double crystal monochromator with two silicon crystal pairs, both the Si(111) and Si(311) for the low and the high energy range, respectively.
The samples were finely ground in an agate mortar with an inert matrix (cellulose), pelletized into disks to optimize the absorption jump of the XANES signal, and then, protected with Kapton tape. The reference samples such as Sb foil (> 95%) and Sb2O3 (99.7%) were purchased from Aldrich and Alfa Aesar, respectively. The reference Sb2O4 and FeSbO4 oxides were prepared as follows: Sb2O4 was obtained by thermal treatment of Sb2O3 powder in air at 600 °C in an alumina boat for 12 h and a temperature ramp of 6 °C/min, the sample was ground and the treatment repeated until a single phase was identified by XRD, of cervantite type. FeSbO4 was prepared by solid-state reaction between FeC2O4·2H2O and Sb2O3, treated at 600 °C in air for 12 h and a ramp of 6 °C/min, then ground and treated at 900 °C in air for 12 h, leading to a single-phase product, identified by XRD, of tripuhyite type (rutile MO2 structure).
Details on data processing
Data processing was performed with OriginPro (V. 8 SR0 and 2018 SR1, OriginLab, Northampton, MA, USA). Crystal structure projections from Figs. 1, 2 and Supplementary Fig. S2 and S3 were generated using Vesta46 graphing tools. Fourier difference density maps in Supplementary Fig. S2 were obtained using GFOURIER 04.06 (Graphic Fourier Program GFOURIER, Version 04.06. Univ. La Laguna, Tenerife, Spain, 2007). Athena and Artemis from the Demeter suite were employed to process the XAFS data31.
References
Nguyen, T. L. et al. Tailored synthesis of antimony-based alloy/oxides nanosheets for high-performance sodium-ion battery anodes. J. Power Sources 414, 470–478 (2019).
Simonin, L., Lafont, U., Tabrizi, N., Schmidt-Ott, A. & Kelder, E. M. Sb/O nano-composites produced via Spark Discharge Generation for Li-ion battery anodes. J. Power Sources 174, 805–809 (2007).
Reddy, M. V., Subba Rao, G. V. & Chowdari, B. V. R. Nano-(V1/2Sb1/2Sn)O4: A high capacity, high rate anode material for Li-ion batteries. J. Mater. Chem. 21, 10003–10011 (2011).
Li, K., Liu, H. & Wang, G. Sb2O3 nanowires as anode material for sodium-ion battery. Arab. J. Sci. Eng. 39, 6589–6593 (2014).
Dragomir, F. et al. Simulation of lithium-ion batteries from a electric vehicle perspective. in 2017 Electric Vehicles International Conference (EV) 1–5 (IEEE, 2017). doi:https://doi.org/10.1109/EV.2017.8242100
Svensson, C. Refinement of the crystal structure of cubic antimony trioxide, Sb2O3. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 31, 2016–2018 (1975).
Sidey, V. On the accurate bond-valence parameters for the Sb3/O2 ion pair. Acta Crystallogr. Sect. B Struct. Sci. 66, 307–314 (2010).
Subramanian, M. A. A., Aravamudan, G., Subba Rao, G. V. & Rao, G. V. S. Oxide pyrochlores—A review. Prog. Solid State Chem. 15, 55–143 (1983).
Mandal, B. P. & Tyagi, A. K. Pyrochlores: Potential multifunctional materials. 8 (2010).
Luca, V., Griffith, C. S., Blackford, M. G. & Hanna, J. V. Structural and ion exchange properties of nanocrystalline Si-doped antimony pyrochlore. J. Mater. Chem. 15, 564 (2005).
Zhu, H. et al. Hydrothermal synthesis and photoluminescence properties of La 2–x Eu x Sn2O7 (x=0–2.0) nanocrystals. J. Am. Ceram. Soc. 90, 3095–3098 (2007).
Sickafus, K. E. Radiation tolerance of complex oxides. Science 289, 748–751 (2000).
Mandal, B. P., Dutta, A., Deshpande, S. K., Basu, R. N. & Tyagi, A. K. Nanocrystalline Nd2–yGdyZr2O7 pyrochlore: facile synthesis and electrical characterization. J. Mater. Res. 24, 2855–2862 (2009).
Matsunami, M., Hashizume, T. & Saiki, A. Ion-exchange reaction of a-site in A2Ta2O6 pyrochlore crystal structure. Arch. Metall. Mater. 60, 941–944 (2015).
Li, N. et al. Enabling pyrochlore-type oxides as highly efficient electrocatalysts for high-capacity and stable Na–O2 batteries: the synergy of electronic structure and morphology. ACS Catal. 7, 7688–7694 (2017).
Sharma, N., Subba Rao, G. V. & Chowdari, B. V. R. Anodic properties of tin oxides with pyrochlore structure for lithium ion batteries. J. Power Sources 159, 340–344 (2006).
Kim, M., Ju, H. & Kim, J. Highly efficient bifunctional catalytic activity of bismuth rhodium oxide pyrochlore through tuning the covalent character for rechargeable aqueous Na–air batteries. J. Mater. Chem. A 6, 8523–8530 (2018).
Oh, S. H., Black, R., Pomerantseva, E., Lee, J.-H. & Nazar, L. F. Synthesis of a metallic mesoporous pyrochlore as a catalyst for lithium–O2 batteries. Nat. Chem. 4, 1004–1010 (2012).
Kim, M., Ju, H. & Kim, J. Single crystalline Bi2Ru2O7 pyrochlore oxide nanoparticles as efficient bifunctional oxygen electrocatalyst for hybrid Na-air batteries. Chem. Eng. J. 358, 11–19 (2019).
Rietveld, H. M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 2, 65–71 (1969).
Stewart, D. J., Knop, O., Ayasse, C. & Woodhams, F. W. D. Pyrochlores. VII. The oxides of antimony: an X-ray and Mössbauer study. Can. J. Chem. 50, 690–700 (1972).
Sidey, V. I., Milyan, P. M. & Semrad, O. O. X-ray rietveld structure refinement of Sb3O6.5. J. Alloys Compd. 490, 598–601 (2010).
Figueiredo, M.-O. Extended vs. local structure in Sb-Pyrochlores: an illustration of the valuable interplay between crystallography and XAFS. in AIP Conference Proceedings882, 205–207 (AIP, 2007).
Mayer, S. F., Falcón, H., Fernández-Díaz, M. T. & Alonso, J. A. The crystal structure of defect KBB′O6 pyrochlores (B, B′: Nb, W, Sb, Te) revisited from neutron diffraction data. Crystals 8, 1–11 (2018).
Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. in Chemische Technologie in Einzeldarstellungen 387–409 (Springer, Berlin, Heidelberg, 1918). doi:https://doi.org/10.1007/978-3-662-33915-2_7
Langford, J. I. & Wilson, A. J. C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 11, 102–113 (1978).
Scheinost, A. C. et al. Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochim. Cosmochim. Acta 70, 3299–3312 (2006).
Gurman, S. J. Interpretation of EXAFS Data. J. Synchrotron Radiat. 2, 56–63 (1995).
Calvin, S. XAFS for Everyone. XAFS for Everyone (CRC Press, 2013). doi:10.1201/b14843
Dalba, G. & Fornasini, P. EXAFS Debye - Waller factor and thermal vibrations of crystals. J. Synchrotron Radiat. 4, 243–255 (1997).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Orosel, D., Balog, P., Liu, H., Qian, J. & Jansen, M. Sb2O4 at high pressures and high temperatures. J. Solid State Chem. 178, 2602–2607 (2005).
Rockenberger, J. et al. Near edge X-ray absorption fine structure measurements (XANES) and extended X-ray absorption fine structure measurements (EXAFS) of the valence state and coordination of antimony in doped nanocrystalline SnO2. J. Chem. Phys. 112, 4296–4304 (2000).
Zou, Y. et al. Pressure-induced anomalies and structural instability in compressed β-Sb2O3. Phys. Chem. Chem. Phys. 20, 11430–11436 (2018).
Svensson, C. The crystal structure of orthorhombic antimony trioxide, Sb2O3. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 30, 458–461 (1974).
Fornasini, P. & Grisenti, R. On EXAFS Debye-Waller factor and recent advances. J. Synchrotron Radiat. 22, 1242–1257 (2015).
Scardi, P., Rebuffi, L., Abdellatief, M., Flor, A. & Leonardi, A. Debye-Waller coefficient of heavily deformed nanocrystalline iron. J. Appl. Crystallogr. 50, 508–518 (2017).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A A32, 751–767 (1976).
Jang, J. & Seung-Joo, K. Photoelectrochemical properties of nanocrystalline Sb6O13, MgSb2O6, and ZnSb2O6-based electrodes for dye-sensitized solar cells. Jpn. J. Appl. Phys. 51, 1–4 (2012).
Rodríguez-Carvajal, J. FULLPROF: A Program for Rietveld Refinement and Pattern Matching Analysis. in Abstracts of Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr 127 (1990).
Wertheim, G. K., Butler, M. A., West, K. W. & Buchanan, D. N. E. Determination of the Gaussian and Lorentzian content of experimental line shapes. Rev. Sci. Instrum. 45, 1369–1371 (1974).
Berar, J. F. & Baldinozzi, G. Modeling of line-shape asymmetry in powder diffraction. J. Appl. Crystallogr. 26, 128–129 (1993).
Finger, L. W., Cox, D. E. & Jephcoat, A. P. Correction for powder diffraction peak asymmetry due to axial divergence. J. Appl. Crystallogr. 27, 892–900 (1994).
Jansen, E., Schaefer, W. & Will, G. R values in analysis of powder diffraction data using Rietveld refinement. J. Appl. Crystallogr. 27, 492–496 (1994).
Simonelli, L. et al. CLAESS: The hard X-ray absorption beamline of the ALBA CELLS synchrotron. Cogent Phys. 3, 1–10 (2016).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Acknowledgments
We are grateful to the Spanish Ministry of Economy and Competitivity for granting the project MAT2017-84496-R, and ILL for making all the facilities available for the neutron diffraction experiments. J.E.R. acknowledges the CAPES agency (Finance Code 001: 88881.171031/2018-01). M.C.A. is greatly ended also to “MATINÉE”, the Associated Unit created by CSIC between the Materials Science Institute of Madrid (ICMM) and the Valencia Institute of Materials Science (ICMUV), Valencia University & CSIC.
Author information
Authors and Affiliations
Contributions
S.M., M.T.F.D., and J.A.A. carried out the growth of the samples and their general structural characterization. J.E.R., C.M., and J.A.A. performed the XAFS experiments. The data processing and analysis have been carried out by J.E.R. (XAFS), S.M., and H.F. (SXRD and NPD). The research design and the manuscript have been written by M.C.A. and J.A.A. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mayer, S.F., Rodrigues, J.E., Marini, C. et al. A comprehensive examination of the local- and long-range structure of Sb6O13 pyrochlore oxide. Sci Rep 10, 16956 (2020). https://doi.org/10.1038/s41598-020-73860-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73860-0
This article is cited by
-
Synergy of diffraction and spectroscopic techniques to unveil the crystal structure of antimonic acid
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.