After reading through Dalton’s atomic theory, you must be wondering what exactly is an atom.
It is just a mere sphere? What is it made of?
Let us see what an atom is.
- It is the smallest particle of an element that retains all chemical properties of the element.
- It usually does not exist individually
-
Size of atom is very small
They are measured in nm (nanometers)
1 nm = 1/1000000000 meters = 10 -9 meters
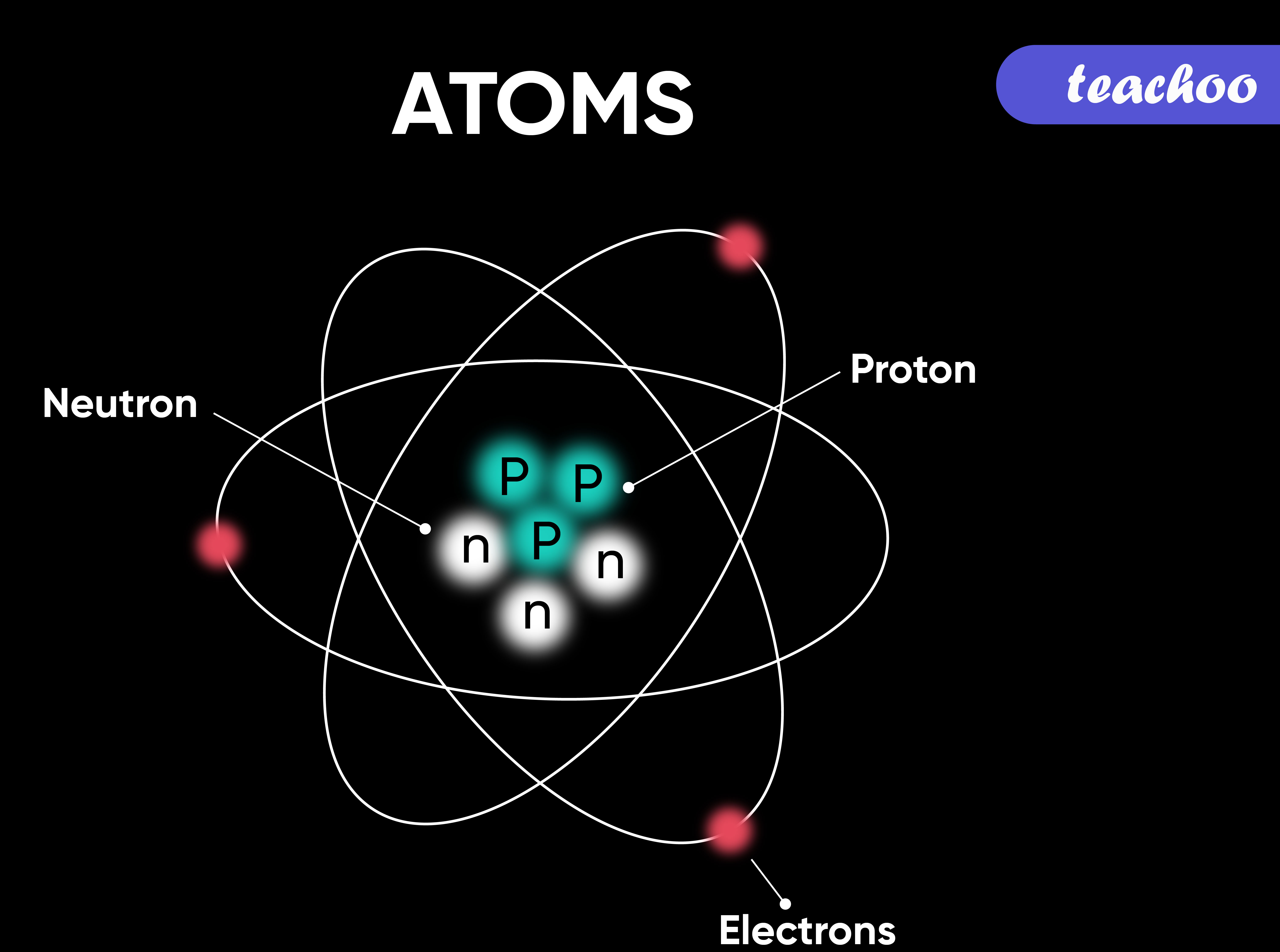
What are Different Components of Atoms?
Atoms can be broken down into Protons, Neutrons and Electrons
- Protons → They are Positively charged
- Electrons → They are Negatively charged
- Neutrons → They have no charge
How do atoms exist?
Atoms normally do not exist independently
They normally exist in the form of Molecules.
Molecules are groups of two or more atoms held together.
Molecules of atoms of the same type are called elements..
What are Elements?
An element is a substance which is made up of only 1 type of atoms
Example
Copper, Carbon, Helium, Hydrogen, Oxygen are all elements

Ancient and modern day symbols of atoms of different elements?
Initially, Dalton used symbols to denote the atoms.
Each symbol did not just denote the name of the atom but also the quantity of atoms.
Each symbol of the atom denoted one of that atom.

In the beginning, the names of elements were derived from the name of the place where they were found for the first time or their colour.
For example
- Copper was named after the place Cyprus.
- Gold was taken from english word meaning yellow.
Now, IUPAC (International Union of Pure and Applied Chemistry) approves names of elements, symbols and units.
1. Most of the symbols are the first one or two letters of the elements’ name in English.
The first letter of a symbol is always written as a capital letter (uppercase) and the second letter as a small letter (lowercase).
For example:
- Symbol of an Oxygen atom is O
- Symbol of Aluminium is Al not AL.
2. Symbols of some atoms are formed from the first letter of the name and a letter appearing later in the name
For Example
- Symbol of Chlorine is Cl
- Symbol of Zinc is Zn

3. Symbols of some atoms have been taken from the names of atoms in Latin, German or Greek.
For Example
- Symbol of Potassium is K which comes from its Latin name Kalium
- Symbol of Iron is Fe which comes from its Latin name Ferrum

Note - Each atom has a UNIQUE name and chemical symbol.
